As of today, the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected more than 108 million individuals and claimed the lives of nearly 2.7 million globally. This past year management of the COVID-19 pandemic solely relied on non-pharmaceutical interventions such as school closures, physical distancing, shielding of those at heightened risk, and self-isolation of symptomatic cases.
Currently, prophylactic vaccines - developed at an unprecedented pace are being administered across the globe. However, many viral variants are emerging, threatening the success of the on-going efforts. Despite the adopted mitigation strategies, the COVID-19 pandemic requires multiple countermeasures.
Nanobodies are a potential therapeutic strategy for treating COVID-19. While many previous studies report nanobodies active against SARS-CoV-2 in vitro, none have been evaluated via animal models' intranasal administration.
Xilin Wu, Lin Cheng, and Ming Fu, et al. designed a novel bispecific format constituted of three nanobodies, trivalent constructed to be tri-valent and bispecific for the RBD (receptor binding domain) of the SARS-CoV-2 spike protein and the human serum albumin (HSA).
Their recent bioRxiv* research paper demonstrates 100% protection in both the prevention and treatment of SARS-CoV-2 infected transgenic hACE2 mice (expressing the human angiotensin-converting enzyme 2) when their engineered nanobodies are administered intranasally.
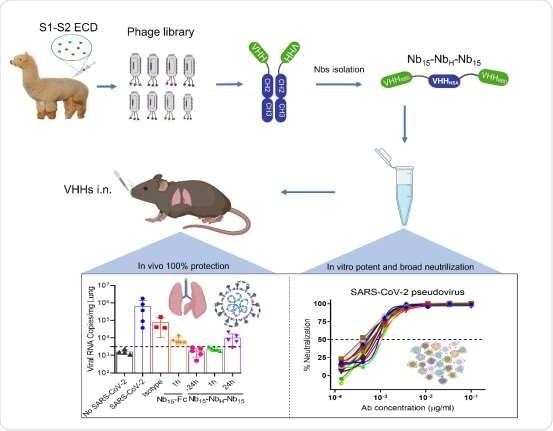
Nb15-NbH-Nb15, with a novel heterotrimeric bispecific configuration, exhibited potent and broad neutralization potency against SARS-CoV-2 in vitro and provided in vivo protection against SARS-CoV-2 infection in hACE2 transgenic mice via intranasal delivery.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
They also report that this novel bispecific antibody exhibited potent inhibitory activity against the wild-type and variants of SARS-CoV-2, including the currently circulating variants, such as the predominant mutant viruses in the UK and South Africa with N501Y mutation.
Engineering nanobodies
Nanobodies (Nbs) are a particular type of antibodies naturally produced by camelids' immune systems, i.e., camels, llamas, and alpacas. Unlike normal antibodies made from 4 proteins bound together: two heavy chains and two light chains, nanobodies are made from just 2 proteins: 2 heavy chains, constituting a single variable domain (Nb) specific for binding antigens. Nanobodies are used as therapeutics agents against viral infection, such as severe fever with thrombocytopenia syndrome virus (SFTSV) and respiratory syncytial virus (RSV).
In this study, an alpaca was immunized with the SARS-CoV-2 spike glycoprotein (S). The researchers isolated the Nbs specific for the RBD from a phage library displaying the Nbs.
Among the lot, they identified three Nbs (3Nb15, Nb15-NbH-Nb15, and Nb15-Fc) that exhibited potent neutralization activity against the live virus and also a panel of SARS-CoV-2 pseudotyped viruses. To improve efficacy and stability, they engineered various configurations of Nbs. Because of the potent neutralization against SARS-CoV-2, the researchers found that the Nb15-NbH-Nb15- a heterotrimer and bi-specific for RBD and HSA, exhibited the most potent neutralization of the virus.
The researchers constructed the Nb consisting of one Nb specific for HSA (human serum albumin) (NbH) to improve the efficacy and the stability in vivo. So, the bi-specific Nbs consisted of one Nb specific for the HSA and the other being specific for RBD. They used (G4S)3, a linker protein to join each Nb.
In vivo pharmacokinetic activity
When administered via i.n. (intranasal), i.p. (intraperitoneal), or i.v. (intravenous), the researchers observed that the three constructs metabolized rapidly. Notably, only upon intranasal administration, the Nb localized in the trachea and lungs. This suggests the intranasal administration be a favorable route for the antibodies to reach the nasopharynx and the lungs where SARS-CoV-2 replicates.
Because the Nb15-NbH-Nb15 sustained for more than 7 days, the researchers chose this construct to avoid any potential adverse drug events in the body. This construct also retained 100% activity after aerosolization, indicating the potential application as a nebulized drug.
Anti-SARS-CoV-2 activity
Nb15-NbH-Nb15 exhibited sub-ng/ml (pM) potency against both the wild-type and the currently circulating mutant variants of SARS-CoV-2, specifically against the variants with the D614G and N501Y mutations that circulate predominantly in the UK and South Africa.
The Nb constructed demonstrated both prophylactic and therapeutic efficacy against SARS-CoV-2 challenge in vivo. The researchers observed superior protection efficacy, especially when the Nbs were given early on during the infection.
Nb15-NbH-Nb15 - ultrahigh neutralization potency
Because significant copies of SARS-CoV-2 are detected in the respiratory tract and a few viral copies in the blood, it is most effective to design direct delivery of therapeutic agents into the tract.
"We suggest that respiratory delivery of Nb15-NbH-Nb15 is a promising route for the prevention and treatment of SARS-CoV-2 infection, and thus warrants further clinical evaluation."
The study here shows that Nb15-NbH-Nb15, with a novel heterotrimeric bispecific configuration, exhibited potent and broad neutralization potency against SARS-CoV-2 in vitro and provided in vivo protection against SARS-CoV-2 infection in hACE2 transgenic mice via intranasal delivery.
Significantly this study demonstrates that the constructed nanobodies show potency against the SARS-CoV-2 variants - D614G and N501Y - which are conferred enhanced replication and transmissibility, emerging as the predominant global variants.
The ultrahigh and broad neutralizing activity of the constructed nanobodies tested in this study - that is both prophylactic and therapeutic - maybe one of the countermeasures we need today.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration, Xilin Wu, Lin Cheng, Ming Fu, Bilian Huang, Linjing Zhu, Shijie Xu, Haixia Shi, Doudou Zhang, Huanyun Yuan, Waqas Nawaz, Ping Yang, Qinxue Hu, Yalan Liu, Zhiwei Wu, bioRxiv 2021.02.08.429275; doi: https://doi.org/10.1101/2021.02.08.429275, https://www.biorxiv.org/content/10.1101/2021.02.08.429275v1
- Peer reviewed and published scientific report.
Wu, Xilin, Lin Cheng, Ming Fu, Bilian Huang, Linjing Zhu, Shijie Xu, Haixia Shi, et al. 2021. “A Potent Bispecific Nanobody Protects HACE2 Mice against SARS-CoV-2 Infection via Intranasal Administration.” Cell Reports 37 (3): 109869. https://doi.org/10.1016/j.celrep.2021.109869. https://www.cell.com/cell-reports/fulltext/S2211-1247(21)01336-X.