Seropositive individuals —people who received antibodies after COVID-19 infection — may be able to safely delay their second mRNA vaccine dose after all, according to the results of a new study.
Increasing immunity is necessary for preventing the spread of SARS-CoV-2. The United States currently leads the world with over 28 million cases and recently reached a sobering milestone of over 500,000 deaths.
The Centers for Disease Control and Prevention (CDC) suggests people who show prior immunity to the virus can delay their vaccination for 90 days to allow more access to other people without any type of immunity.
The messenger RNA (mRNA) vaccines were designed to help individuals prepare the immune system to detect and create antibodies towards the spike protein of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, it remains unknown how the vaccine responds to people who already have antibodies in their system.
Allison E. Aiello and colleagues from the University of North Carolina worked with the COVID HCP Study Team to study the immune response of people who receive the vaccine versus those who receive it naturally after infection.
The researchers write:
“Our results provide preliminary evidence that prior SARS-CoV-2 infection may prime the response to the first mRNA-based SARS-CoV-2 vaccine dose. These findings could have a significant impact on the allocation of mRNA-based vaccines.”
The study “SARS-CoV-2 seropositivity after infection and antibody response to mRNA-based Vaccination” is available as a preprint on the medRxiv* server, while the article undergoes peer review.
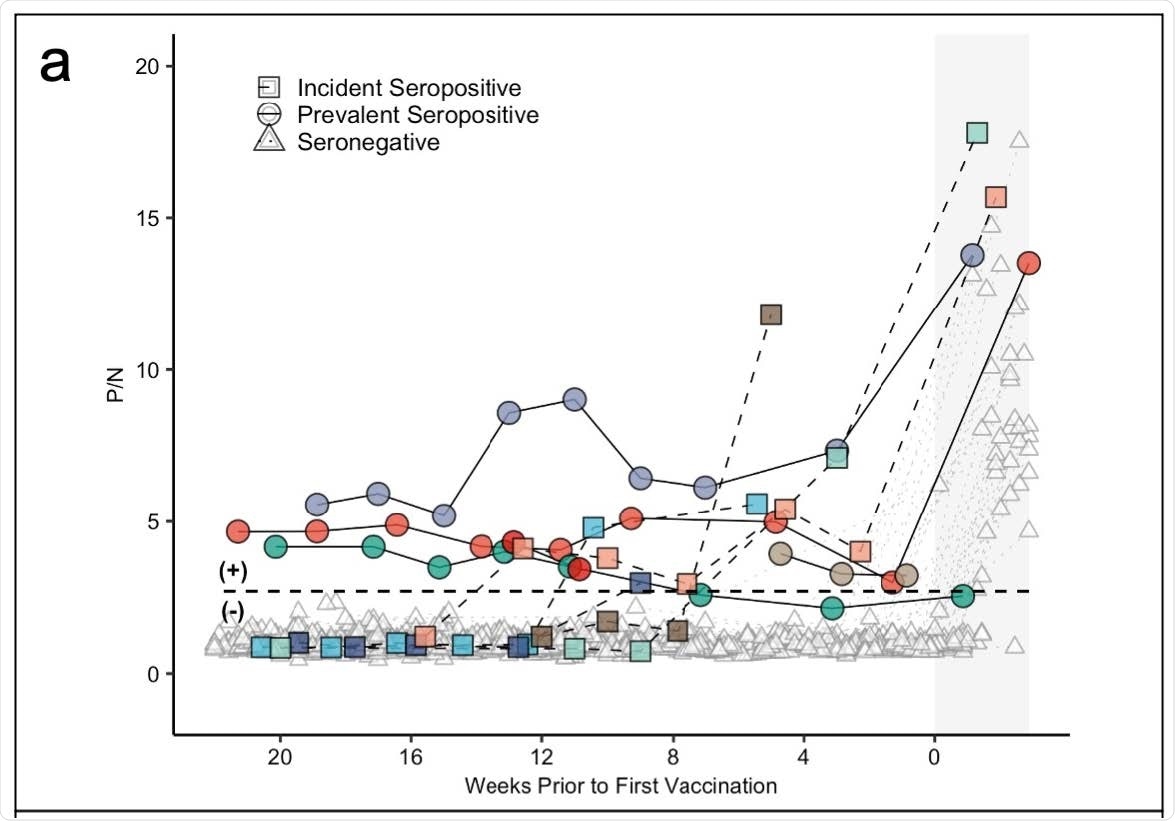
Panel (a) demonstrates antibody levels over time in SARS-CoV-2 seronegative (triangles) and seropositive individuals, including those who were seropositive at enrollment (prevalent seropositive, circles) and those who became seropositive during follow-up (incident seropositive, squares). The dotted line is a P/N ratio of 2.57, the cut-off associated with 99.5% specificity (SARS-CoV-2 Ig-positive above the line, Ig-negative below). The x-axis represents weeks to first vaccine dose; values post-vaccine 1 are shaded.
Measure changes in antibody levels
The team began enrolling healthcare workers from an academic medical center into a longitudinal observational cohort study in July 2020. From February 17, 2021, the study had enlisted 213 healthcare workers, with 91% providing at least one blood sample.
The goal was to observe changes in antibody levels over time.
Blood samples were taken every two weeks for the first 12 weeks and then every month. The team also measured the amount of immunoglobulin antibodies that were specific to the receptor-binding domain of the SARS-CoV-2 spike protein present in blood serum before and after vaccination from either the Moderna or Pfizer vaccine.
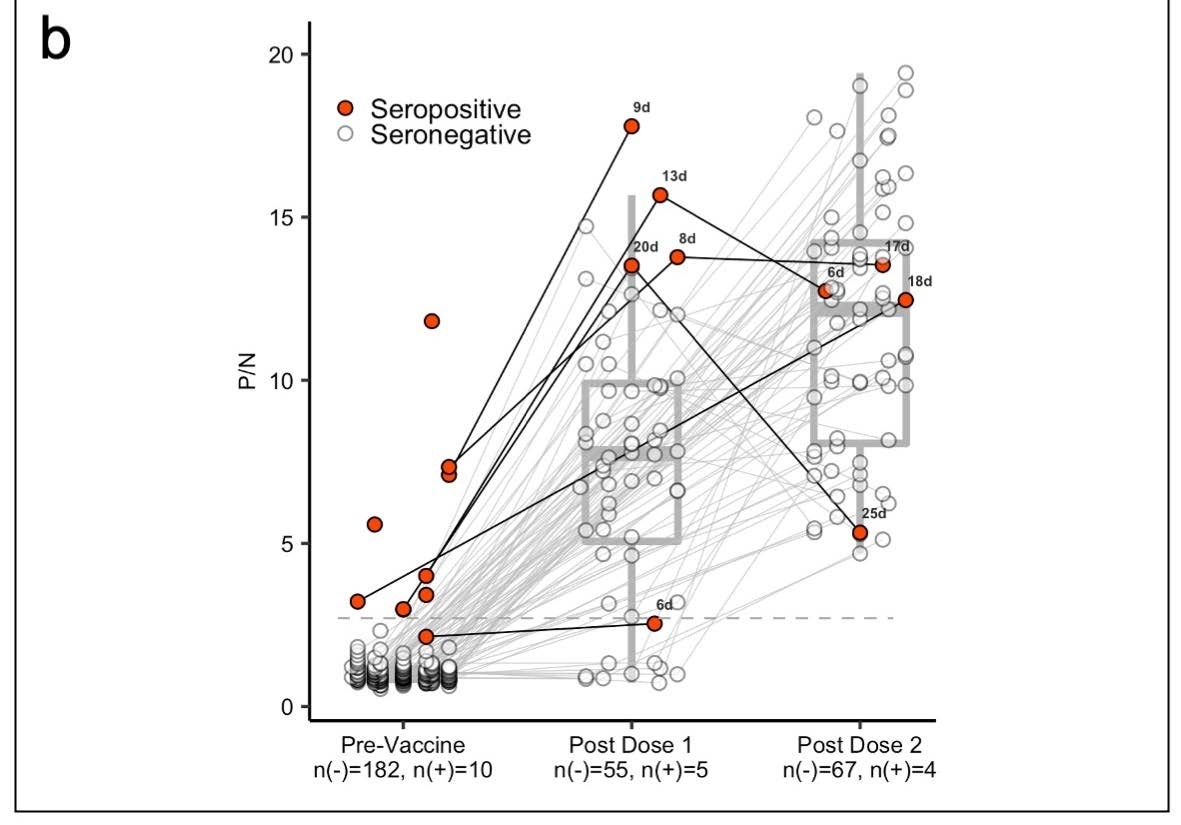
Panel (b) shows box plots with the medians and interquartile ranges of P/N ratios pre-vaccination, post-vaccine 1, and postvaccine 2 by serostatus (seronegative: n(-), seropositive: n(+)).The upper and lower whiskers extend to the largest and smallest values no further than 1.5 times IQR from the hinge, respectively. Data beyond the end of the whiskers are outliers. For the pre-vaccine time point, the most recent antibody level prior to vaccination (for those who were vaccinated) or most recent antibody level overall (for those who were not vaccinated) is shown. For the post-vaccine time points, the first measurement after 5 days post-vaccination is included.
Seropositive patient information
Results showed 3%, precisely five participants were classified as ‘prevalent seropositive’ at the beginning of the study.
One individual continued to show seropositivity for 6 months and another for 3 months — in the absence of a vaccine. Although both showed no antibody levels, they were seronegative one month before they got vaccinated.
Five people became seropositive through the study. Approximately 4 of the 5 individuals were symptomatic for COVID-19 infection before becoming seropositive.
Boost in antibody response in seropositive individuals after the first vaccination
About 97% of study participants received at least one dose of the Moderna or Pfizer vaccine. The team was able to measure the antibody levels of 6 of the 10 (60%) seropositive individuals and 87 of the 183 (48%) seronegative individuals.
Results showed people who were seropositive to SARS-CoV-2 had a two-fold increase in antibody response after their first mRNA dose compared to people who were seronegative.
Additionally, seropositive individuals showed antibody levels similar to seronegative individuals who received two doses of the vaccine.
Future directions
The study provided preliminary results given the small sample of seropositive participants compared to seronegative participants. The authors suggest replicating the study’s findings would bring more insight into “the durability of the response to the first dose of SARS-CoV-2 vaccine among previously infected individuals.”
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.