The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) invades host cells through ACE2 receptors found on the surface of human cells. Angiotensin-converting enzyme 2 (ACE2) receptors are abundant and found in several body areas, including the nose, kidneys, heart, and lungs.
SARS-CoV-2 targeting of ACE2 receptors has caused damage to multiple organs in the body, contributing to several long COVID symptoms. Based on previous autopsy research, evidence suggests the virus can target ACE2 receptors in adult female ovaries.
A team of scientists from China collaborated to find if SARS-CoV-2 could also infect the ovary at different developmental stages.
The researchers write:
"We found that primordial germ cells and cumulus-oocyte complex exhibited high ACE2 expression. More importantly, the ratio of ACE2-positive cells was sharply up-regulated in primary stage and ACE2 was expressed in all oocytes and cumulus cells in preovulatory stage, suggesting the possible risk of SARS-CoV-2 infection in follicular development."
Their findings suggest developing ovaries could be a potential target for SARS-CoV-2 infection.
The study "Comprehensive evaluation of ACE2 expression in female ovary by single-cell RNA-seq analysis" is available as a preprint on the bioRxiv* server, while the article undergoes peer review.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
How they did it
The team used previous single-cell RNA-sequencing datasets to create a developmental-stage-specific risk map of SARS-CoV-2 infection that included infection risk in fetal primordial germ cells, the cumulus-oocyte complex, and the adult ovary.
Cells with ACE2 expression were labeled ACE2-positive cells. High-risk cell types have deemed any cell with an ACE2-positive proportion >1%.
The expression level of ACE2 in AT2 cells in the lungs was used for comparison purposes.
Fetal primordial germ cells at high-risk for viral invasion
There are four stages of development to create fetal primordial germ cells, and ACE2 expression was found in all four cell types. Mitotic cell types had 3% ACE-2 positive cells, RA signaling-responsive phase had 10% ACE2-positive cells, and meiotic prophase had 10% ACE2-positive cells.
The last developmental phase is the oogenesis phase which showed the highest ACE2 expression with 59% ACE2-positive cells. Since all cell types had more than 1% of ACE2-positive cells, the authors suggest primordial germ cells are at high risk for coronavirus infection.
Investigating further, they found that CatB/L expression in ACE2 positive primordial germ cells could help SARS-CoV-2 infect these cells. This was not observed in nearby somatic cells that do express CatB/L, which suggests a low risk of SARS-CoV-2 infection.
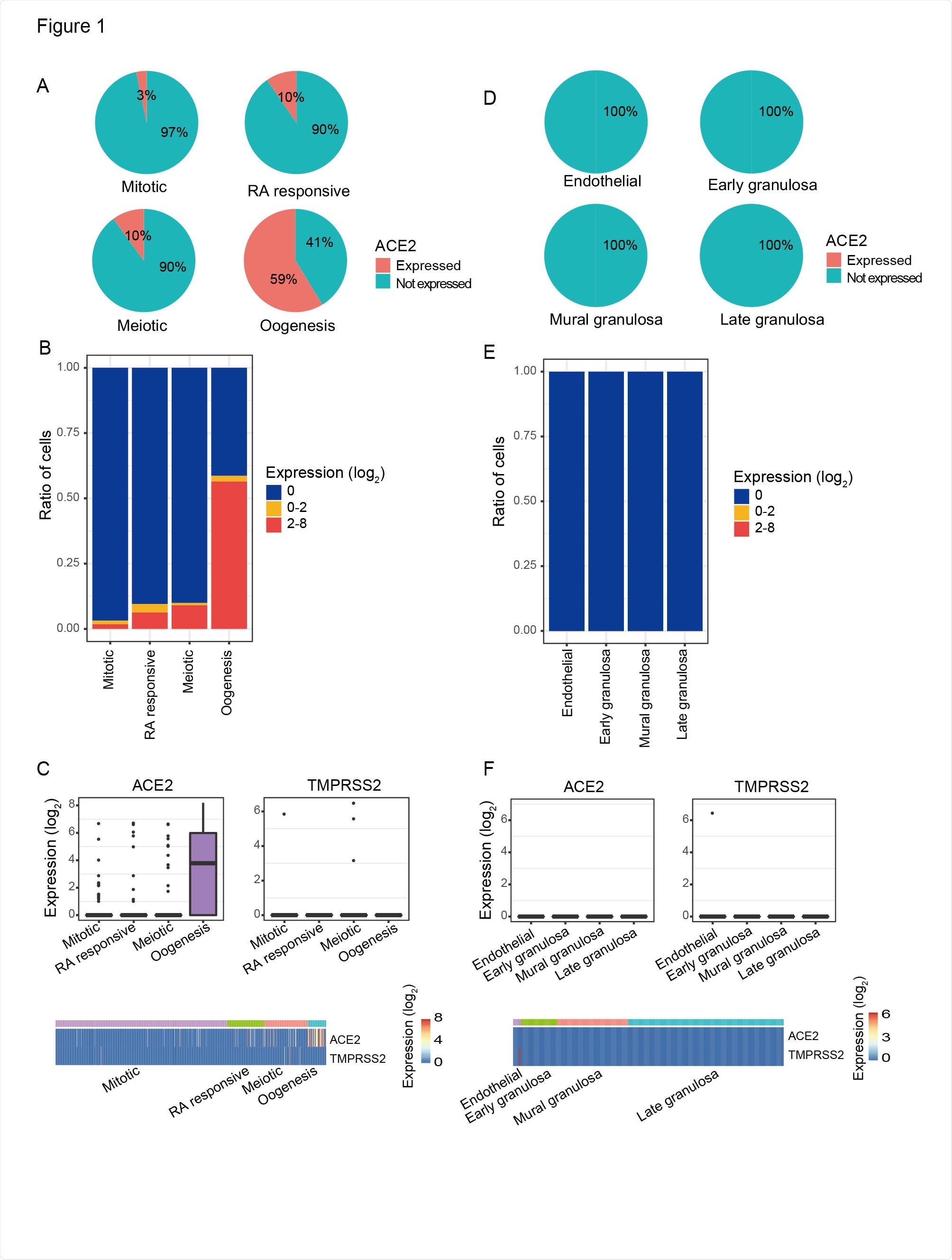
The ACE2 expression pattern in female PGCs and their surrounding somatic cells. A) Pie charts showed the ratio of ACE2 expressed PGCs in each developmental stage. Mitotic, RA responsive, Meiotic, and Oogenesis represent four sequentially developmental stages of female PGCs. B) Ratios of different ACE2 expression level of PGCs in each developmental stage. C) ACE2 and TMPRSS2 expression level of PGCs. Top: boxplots of ACE2 and TMPRSS2 expression level in each developmental stage. Bottom: heatmaps of ACE2 and TMPRSS2 expression level in each cell. D) Pie charts showed the ratio of ACE2 expressed PGC surrounding somatic cells. Endothelial: somatic cells surrounding the PGC in Mitotic stage; Early granulosa: somatic cells surrounding the PGC in RA responsive stage; Mural granulosa: somatic cells surrounding the PGC in Meiotic stage; Late granulosa: somatic cells surrounding the PGC in Oogenesis stage. E) Ratios of different ACE2 expression level of PGC surrounding somatic cells. F) ACE2 and TMPRSS2 expression level of PGC surrounding somatic cells. Top: boxplots of ACE2 and TMPRSS2 expression level in PGCs surrounding somatic cells. Bottom: heatmaps of ACE2 and TMPRSS2 expression level in each cell.
High ACE2 expression in oocytes
As primordial germ cells develop into immature oocytes, the team next looked at ACE2 folliculogenesis and found high ACE2 expression in all five developmental stages.
The least amount of ACE2 was found 52.9%, and found in ACE2-positive cells of the primordial follicles. In contrast, there was a 100% ACE2 expression in oocytes that had crossed from the antral to the preovulatory stage.
Similar to what's observed with primordial germ cells, oocytes also expressed high amounts of CTSB and CTSL, suggesting CatB/L could help with viral entry into cells.
"Taken together, oocytes during follicular development may be at high risk of SARS-CoV-2 infection, especially in antral and preovulatory stage," wrote the researchers.
Purpose of ACE2 expression in oocyte maturation
The authors suggest the higher ACE2 expression in later stages could imply a developmental role. "Moreover, the ratio of ACE2-positive cells was sharply up-regulated in primary follicles, indicating that ACE2 may play a crucial role in this stage."
"Besides, we found that the proportion of ACE2-positive cells was sharply up-regulated in oocytes at primary stage and ACE2 may be related to metabolic process and regulation of autophagy," wrote the researchers. "In addition, cumulus cells were also found to be related to receptor recognition and compound transport. These reminded us that ACE2 changes might have influences on oocyte maturation."
Using a Gene Ontology analysis of the primary stage, the researchers examined what ACE2 does in developing ovaries.
They found that ACE2-positive cumulus cells with up-regulated genes are associated with a signaling pathway involving receptor recognition and compound transport.
Down-regulated genes in ACE2-positive oocytes showed involvement in cilium movement.
The research team concludes that the high ACE2 expression present in an embryo's developing stages can increase the risk for SARS-CoV-2 infection. In addition, catB/L expression could help with SARS-CoV-2 entering and infecting a host cell.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.