COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), affects people differently. While some people show no symptoms, others have a severe and often fatal version of the disease. Pre-existing medical conditions, being male, and being immunocompromised are some factors that affect disease outcomes.
There is also concern that people with asthma will have an increased risk for COVID-19, given previous experience with other respiratory viruses that worsen asthma. However, some studies have found that the risk of being infected and hospitalized for COVID-19 is lower in people with asthma. Many factors, such as being careful to limit virus exposure, younger age, and other biological features, could provide protection.
The airway epithelium, a key SARS-CoV-2 infection site, is modified in people with asthma, with changes due to the cytokine Interleukin 13 (IL-13) observed in about half the patients. IL-13 decreases the expression of the angiotensin-converting enzyme 2 (ACE2), the SARS-CoV-2 receptor in humans, and is known to protect against other RNA viruses.
Testing effect of IL-13 on SARS-CoV-2 infection
In a paper published on the bioRxiv* preprint server, researchers reported the results on whether IL-13 expression in asthma patients reduces their susceptibility to SARS-CoV-2 infection.
The team used primary human bronchial epithelial cells (HBEC) from 13 individuals and they cultured the cells with and without IL-3, and then infected them with the SARS-CoV-2 virus.
From a list of 342 SARS-CoV-2 associated genes, the team found 332 of these genes in HBEC cultures without IL-13. Further analysis revealed that genes were expressed differently in the different cell types. IL-13 increased TMPRSS2 expression in secretory cells but decreased it in ciliated cells. This different expression could affect infection outcomes in the different epithelial cells.
The team then investigated if the SARS-CoV-2 genes were modified in asthma using data from asthmatic and healthy individuals using the three gene metric (TGM), a common method for measuring IL-13 induced airway inflammation in asthma.
The team found 24 of the 27 SARS-CoV-2 associated genes induced by IL-13 were positively correlated with the TGM and 16 of these were significantly associated with the TGM when age and sex were included. The results indicate SARS-CoV-2 associated genes induced by IL-13 are similar to those seen in people with asthma or chronic obstructive pulmonary disease (COPD).
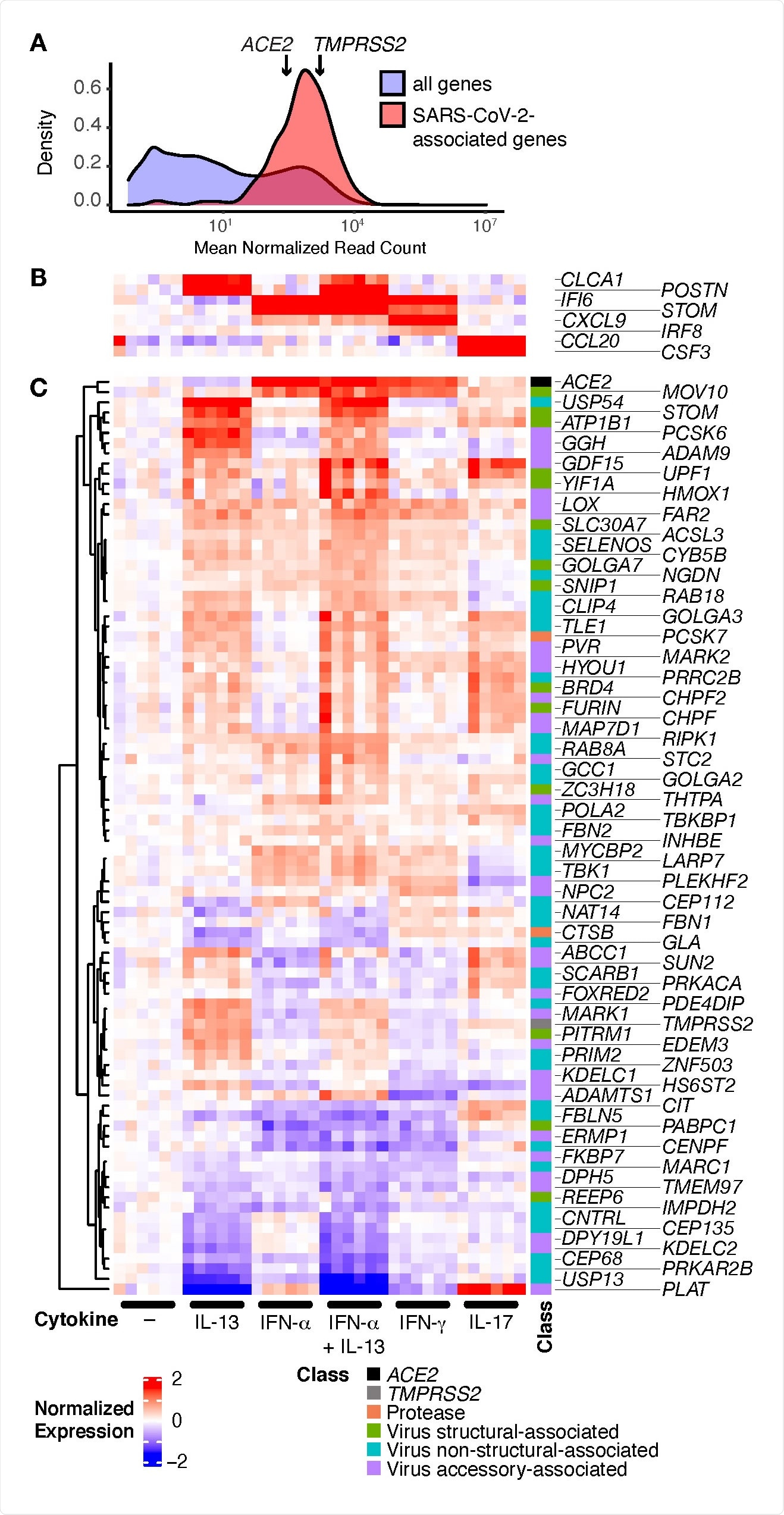
SARS-CoV-2-associated genes are highly expressed in HBECs and many are regulated by cytokines. HBECs from six donors were cultured without cytokine (–), or with IL 13, IFN-α, a combination of IL-13 and IFN-α, IFN-γ, or IL-17 and analyzed by RNA-seq. (A) Comparison of read counts between SARS-CoV-2 associated genes, including ACE2 and TMPRSS2, and all detected genes (≥1 read per million mapped reads in ≥50% of samples) in unstimulated HBECs. (B, C) Heatmap illustrating canonical cytokine-regulated genes (B), and cytokine regulated SARS-CoV-2-associated genes (C; FDR q ≤ 0.05; absolute fold change ≥ 1.5 for any cytokine).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
When the team tested for viral RNA in the HBEC cells infected with SARS-CoV-2 after 48 hours, they found pre-stimulation with IL-13 significantly decreased viral infection by more than 95%. In addition, the presence of mucus decreased viral RNA detected in unstimulated cells by 74% compared to cells without mucus.
Next, the authors tested for the presence of double-stranded DNA (dsDNA), produced during viral replication and how it is affected by mucus and IL-13. They did not see any dsDNA in IL-13 stimulated cultures, but small amounts of viral RNA was detected. This could be because the dsDNA test is less sensitive than qRT-PCR or IL-13 totally prevented viral replication, and the RNA detected is remnants of viral inoculum.
IL-13 reduces SARS-CoV-2 infection
Since many of the modifications due to IL-13 on the SARS-CoV-2 associated genes were seen in cultured cells as well as samples obtained from individuals with type 2 asthma, this could be why people with asthma may be protected from COVID-19. The researchers also found associations between the genes and type 2 inflammation in smokers with and without COPD.
The effect of IL-13 on the genes was different from that of IFN-a, likely because they induce different antiviral mechanisms. The experiments suggested not only IL-13, but the mucus gel may also protect against infection. “While effects of IL-13 on the airway epithelium are an important contributor to asthma pathogenesis, it is intriguing to speculate that IL-13 responses may have evolved at least in part to protect against viral infections,” write the authors.
Several different mechanisms may be responsible for SARS-CoV-2 inhibition by IL-13. A previous study showed a single cytokine could activate many antiviral pathways. The decrease in ACE2 expression by IL-13 could be a possible mechanism. The results also showed mucus helped prevent infection. IL-13 also regulates mucins, glycoproteins that form mucus, forming mucus gel on the epithelium.
Although the exact mechanism of IL-13 inhibiting SARS-CoV-2 is still unclear, understanding the antiviral pathways may help develop treatments for COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Bonser, L. R. et al. (2021) The type 2 asthma mediator IL-13 inhibits SARS-CoV-2 infection of bronchial epithelium. bioRxiv. https://doi.org/10.1101/2021.02.25.432762, https://www.biorxiv.org/content/10.1101/2021.02.25.432762v1
- Peer reviewed and published scientific report.
Bonser, Luke R., Walter L. Eckalbar, Lauren Rodriguez, Jiangshan Shen, Kyung Duk Koh, Khadija Ghias, Lorna T. Zlock, et al. 2022. “The Type 2 Asthma Mediator IL-13 Inhibits Severe Acute Respiratory Syndrome Coronavirus 2 Infection of Bronchial Epithelium.” American Journal of Respiratory Cell and Molecular Biology 66 (4): 391–401. https://doi.org/10.1165/rcmb.2021-0364oc. https://www.atsjournals.org/doi/10.1165/rcmb.2021-0364OC.