Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus behind the coronavirus disease 2019 (COVID-19) pandemic, has claimed over 2.54 million lives so far and affected more than 114 million people globally. In the U.S. alone, more than 516,000 deaths are attributed to the virus. Neutralizing antibodies targeting the SARS-CoV-2 spike protein are the most widely used candidates for developing vaccines and therapeutics. So far, there have been three Emergency Use Authorizations by the U.S. Food and Drug Administration for such antibodies and combinations, and many others are in the development pipeline.
Studies have shown that the inflammatory phase of COVID-19 is associated with severe disease and acute respiratory distress syndrome. During this phase, macrophages and monocytes are activated, and cytokines are released, leading to a cytokine storm. It may also involve viral transmission independent of the ACE2 receptor. Those with activated immune and endothelial cells may also be more sensitive to inflammation associated with the antibody-virus immune complex. However, treatment benefit with monoclonal antibodies has only been observed in trials involving outpatients with mild to moderate disease but not in hospitalized patients with advanced disease.
Determining the timing of anti-SARS-CoV-2 antibody production in hospitalized patients
To address this gap, researchers from the U.S. evaluated the timing of antibody production against SARS-CoV-2 in hospitalized patients. They used a highly sensitive multiplexed bead-based immunoassay that allows for early detection of anti-SARS-CoV-2 antibodies. The study is published on the preprint server medRxiv*.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The study cohort had 33 patients, with 11 females and 22 males ranging from 30 to 95 years. There were 4 to 16 collections per patient over 0 to 42 days after onset of symptoms. Antibody levels for the SARS-CoV-2 antigens were plotted against time based on days after symptom onset in the discharged and expired patients.
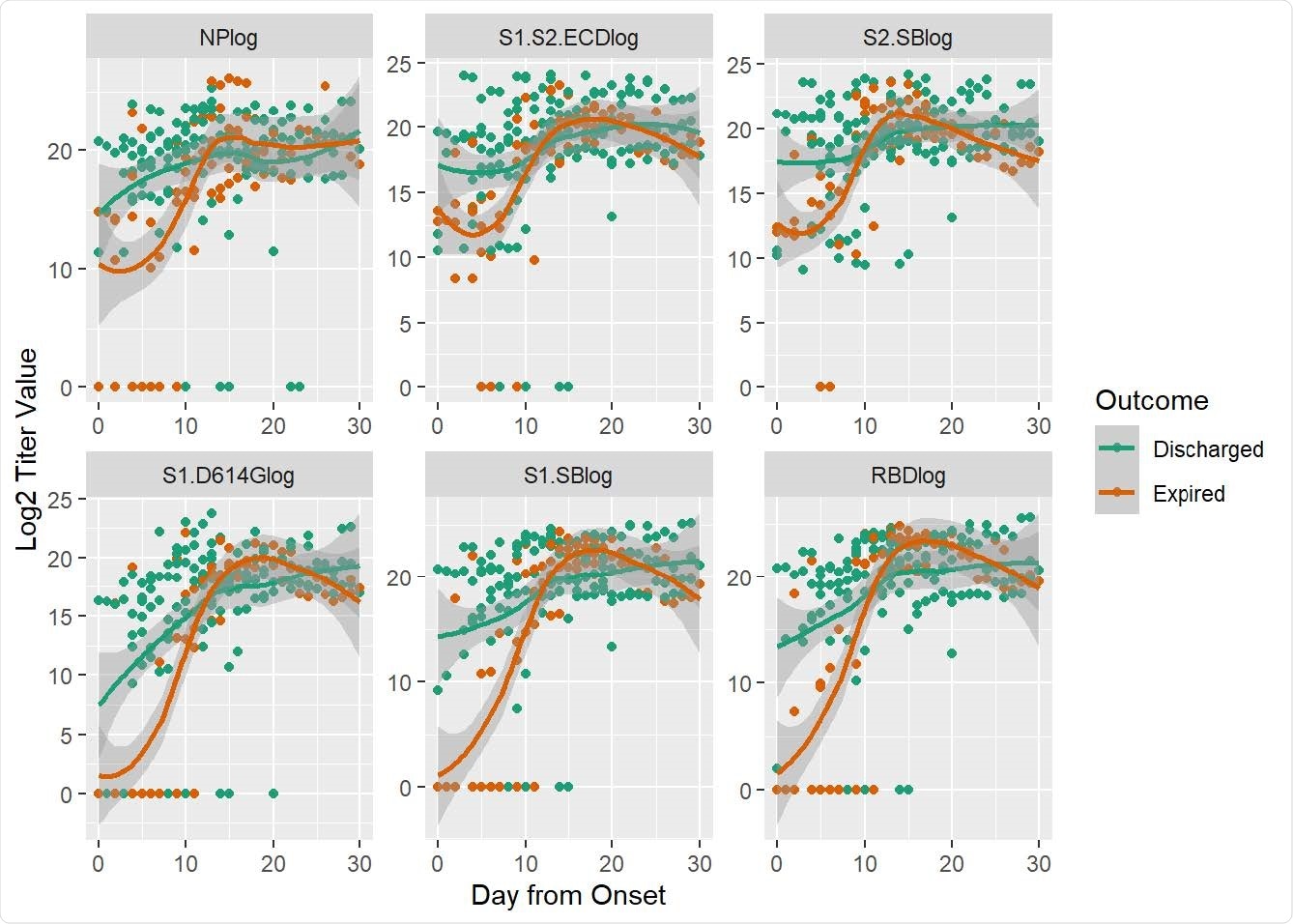
Antibody titers at different days after onset were measured by the multiplexed beads array as described in Methods. Displayed are specific IgG antibody titer values (log transformed) in expired (orange) and discharged (green) groups of patients targeting the nucleocapsid protein (NP) and five spike protein components, i.e., S1+S2 ECD, S2, S1 (D614G), S1 and RBD. Dots are titer values for each patient at the corresponding day from onset; lines are smoothed regression fit to the observed data with 95% confidence interval bands.
Expired COVID-19 patients had significantly lower anti-SARS-CoV-2 antibody levels compared to discharged patients
The researchers found that patients who expired had significantly lower antibody levels to the SARS-CoV-2 spike protein in the first week after onset of symptoms compared to discharged COVID-19 patients. They also developed a model to predict COVID-19 outcomes based on antibody level trajectory. A notable difference was observed in outcome by sex – Only 1 female in the cohort expired compared to 10 in the male cohort.
“Although the mechanisms for these findings need further elucidation, these results align well with clinical studies on therapeutic antibodies.”
This study shows that early detection of antibodies against SARS-CoV-2 was associated with better survival in hospitalized COVID-19 patients. Early levels of antibodies predicted COVID-19 outcome, and this result is consistent with therapeutic antibody benefit early in the COVID 19 disease course. This model is supported by convalescent plasma therapy that shows better outcomes associated with early treatment and lack of benefit with late treatment.
“Early antibody responses may relate to variability in time of infection to symptom onset and reflect other mechanisms related to disease onset”.
Sensitive assay methods and proper modeling could help predict clinical course and choose appropriate therapeutic interventions
The findings show that while developing an early antibody response as an outcome predictor, it is crucial to have a sensitive method such as the flow cytometry bead-based method used in this study to detect antibodies early in the course of disease reliably. Such an assay method with proper modeling could help predict patients’ clinical course and select appropriate therapeutic interventions.
According to the authors, the exact stage of disease progression that may benefit from antibody therapy needs to be studied further. It may depend on the nature of the antibodies and additional interventions. With more and more studies on neutralizing antibodies, early antibody levels may help to decide appropriate therapies in the future.
“While the above approaches helped us demonstrate significant differences in the early antibody response between patients who expired and those who were discharged, it did not tell us whether the antibody response trajectory itself can be predictive of the eventual outcome.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Early Antibody Responses Associated with Survival in COVID19 Patients Zhao-Hua Zhou, Sai Dharmarajan, Mari Lehtimaki, Susan L. Kirshner, Steven Kozlowski, medRxiv, 2021.02.21.21252168; doi: https://doi.org/10.1101/2021.02.21.21252168, https://www.medrxiv.org/content/10.1101/2021.02.21.21252168v1
- Peer reviewed and published scientific report.
Zhou, Zhao-Hua, Sai Dharmarajan, Mari Lehtimaki, Susan L. Kirshner, and Steven Kozlowski. 2021. “Early Antibody Responses Associated with Survival in COVID19 Patients.” Edited by Kanta Subbarao. PLOS Pathogens 17 (7): e1009766. https://doi.org/10.1371/journal.ppat.1009766. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009766.