As the coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues, many countries have rolled out vaccines against the virus. In an encouraging preprint research paper posted to the medRxiv* server, scientists report on the vaccine efficacy in the Danish vaccination program.
The campaign in Denmark commenced at the end of 2020, and used the Pfizer/BioNTech BNT162b2 vaccine. BNT162b2 is built on the messenger ribonucleic acid (mRNA) platform, and encodes the immunodominant viral spike antigen.
The first round of vaccination prioritized residents of long-term care facilities and healthcare workers (HCWs) working on the frontline. The current paper deals with estimated vaccine efficacy (VE) in these two groups.
Study details
The researchers performed a retrospective study based on all care home residents and HCWs, using a polymerase chain reaction (PCR) test to diagnose the presence of SARS-CoV-2 infection. They then estimated the VE after the first and the second dose at varying time points.
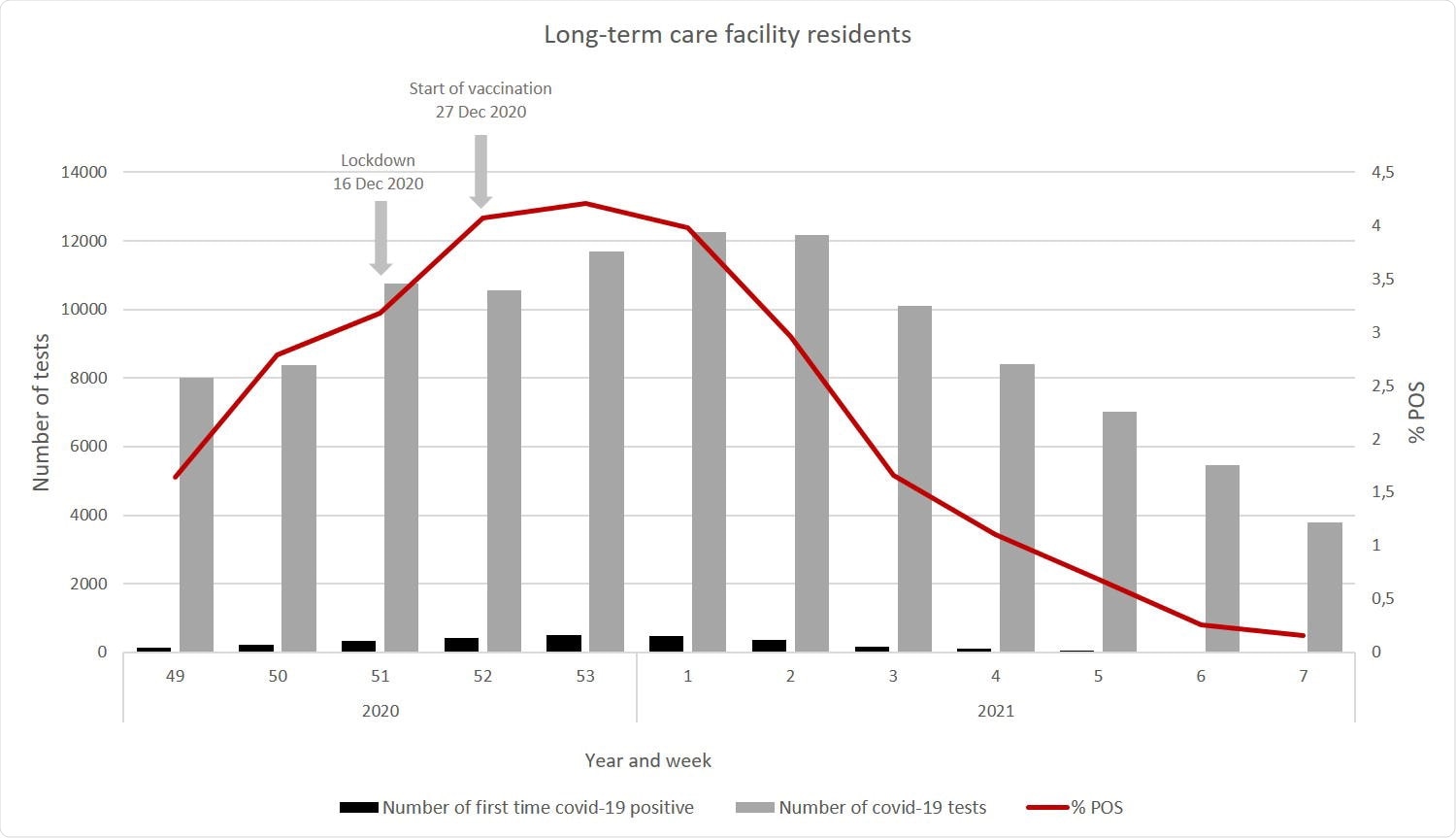
The study included over 39,000 care home residents and over 3,31,000 HCWs. The median age of the former was 84 years, and of the latter, 47 years. About 95% of care home residents received the first dose of the vaccine, and 86% the second dose, between December 27, 2020, and February 18, 2021.
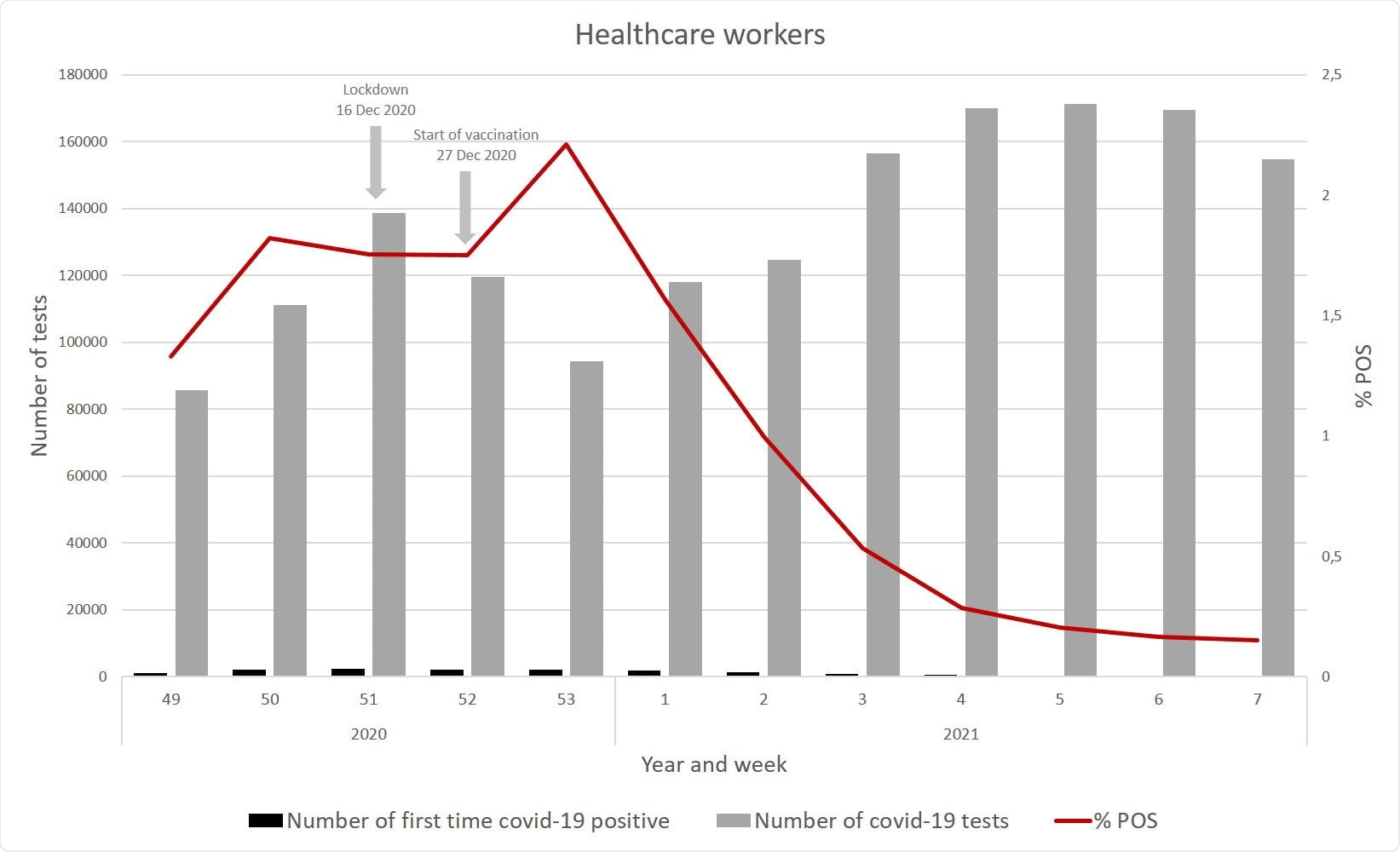
Among HCWs, about 28% and 24% received the first and second doses during this period.
The vaccine used for 99% of care home residents, and ~90% of HCWs, was the BNT162b2 mRNA vaccine.
What are the results?

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Follow-up was continued for a median of 53 days. The researchers found that the proportion of cases among vaccinated care home residents after one dose was not significantly different from that among the unvaccinated in the same group.
However, during the first week after the second dose, there were 57 cases of COVID-19 among care home residents during this period, and 52 in the HCW group. Beyond this period, there were 27 and 10 cases, respectively.
The total number of infections among care home residents after two doses of the BNT162b2 vaccine was therefore 84. Overall, there were ~1,500 cases during this period among care home residents and over 7,000 among HCWs.
In comparison, there were almost 490 infections among unvaccinated care home residents and over 5,660 among unvaccinated HCWs.
Thus, the first dose showed no protective efficacy among care home residents. Among HCWs, the VE at 14+ days from the first dose but before the second dose was 17%.
When evaluated at seven days following the second dose, VE was 52% and 46% in care home residents and in HCWs, respectively. After this period, it went up to 64% and 90% in these groups.
These results are obtained after adjusting for the effects of sex, age and other medical conditions.
What are the implications?
The scientists found hope in these results, which showed high VE soon after administering the second dose of the BNT162b2 vaccine. This study is extremely useful as it is among the earliest to report the real-life VE of this vaccine after two doses. Earlier studies have been limited to reporting the interim VE after the first dose.
The VE of 90% and 64% after two doses, among care home residents and HCWs, respectively, strengthens the case for providing this regimen worldwide. The elderly, who make up a large proportion of care home residents, are especially at risk, especially as they often have multiple comorbidities.
Moreover, the weakened immune system among the elderly is a cause for doubt about the efficacy of vaccination in this group. Here again, the VE of 64% indicates the utility of this vaccine in protecting the elderly against the severity of COVID-19.
The national incidence of COVID-19 was at its peak when the vaccine program began, and many care home residents were being infected. As a result, a partial lockdown was implemented in the region a few weeks prior to the immunization program. Therefore, the unadjusted VE was higher than the final results, which could be in part due to the effects of this measure.
In addition, the high rate of vaccination among care home residents may have led to an overestimation of the observed VE, since the infection risk would have been sharply reduced by the high vaccine coverage within this closed setting.
While these findings corroborate other studies that indicate the importance of a two-dose schedule with BNT162b2, a more extended period of follow-up between the prime and boost dose could help to elicit the full protective impact of a delayed-second-dose regimen.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.