Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in late December 2019 in Wuhan, China. The virus has since spread across the globe, evolving into the COVID-19 pandemic, crippling global public health systems and economies. The scientific community sprang into action and made unprecedented strides in vaccine research and development, leading to the generation of multiple vaccines for COVID-19, utilizing various approaches. However, SARS-CoV-2 is parallelly evolving into newer variants with different levels of infectivity and viral immune evasion.
Using advanced genomics, scientists were able to identify thousands of circulating SARS-CoV-2 variants with several receptor-binding domain (RBD) mutations made possible through natural selection. The variability of RBD epitopes is concerning since these mutations can decrease the efficacy of vaccines and increase viral transmissibility making the variant more infectious.
It is vital to track and study RBD mutants because of the role of the RBD: ACE2 interaction site in SARS-CoV-2 transmission and neutralization, as well as their increased transmissibility and lethality. Tracking of RBD mutants has helped identify several SARS-CoV-2 variants such as the Mink, UK, South African, and LA variants.
Research so far has focused on the protection offered by vaccination or infection against the RBD mutants in newly emerging SARS-CoV-2 variants. Despite increasing data provided by companies that develop these vaccines, it is unclear if the vaccines designed to act against the original Wuhan strain of SARS-CoV-2 will be effective against emerging viral variants such as the UK variant or the South African variant.
Characterizing the antibody response to Pfizer BNT-162b2 vaccine and investigating the efficacy of neutralizing antibodies against newer variants
Researchers from Germany and the US investigated the efficacy of neutralizing antibodies against newer variants using serum and saliva samples obtained from groups of infected, vaccinated (with Pfizer BNT-162b2), and uninfected individuals. The researchers started this study to uncover vaccine-induced immune response and show the efficiency of antibody binding and neutralization in SARS-CoV-2 variants. The study is published on the preprint server, medRxiv*.
Antibody responses, including salivary antibody response and antibody binding to RBD mutant strains in these groups, were analyzed. The neutralization capacity of the antibody response against a South African variant isolated from a patient was tested with the help of viral neutralization tests and then verified by an ACE2 competition assay.
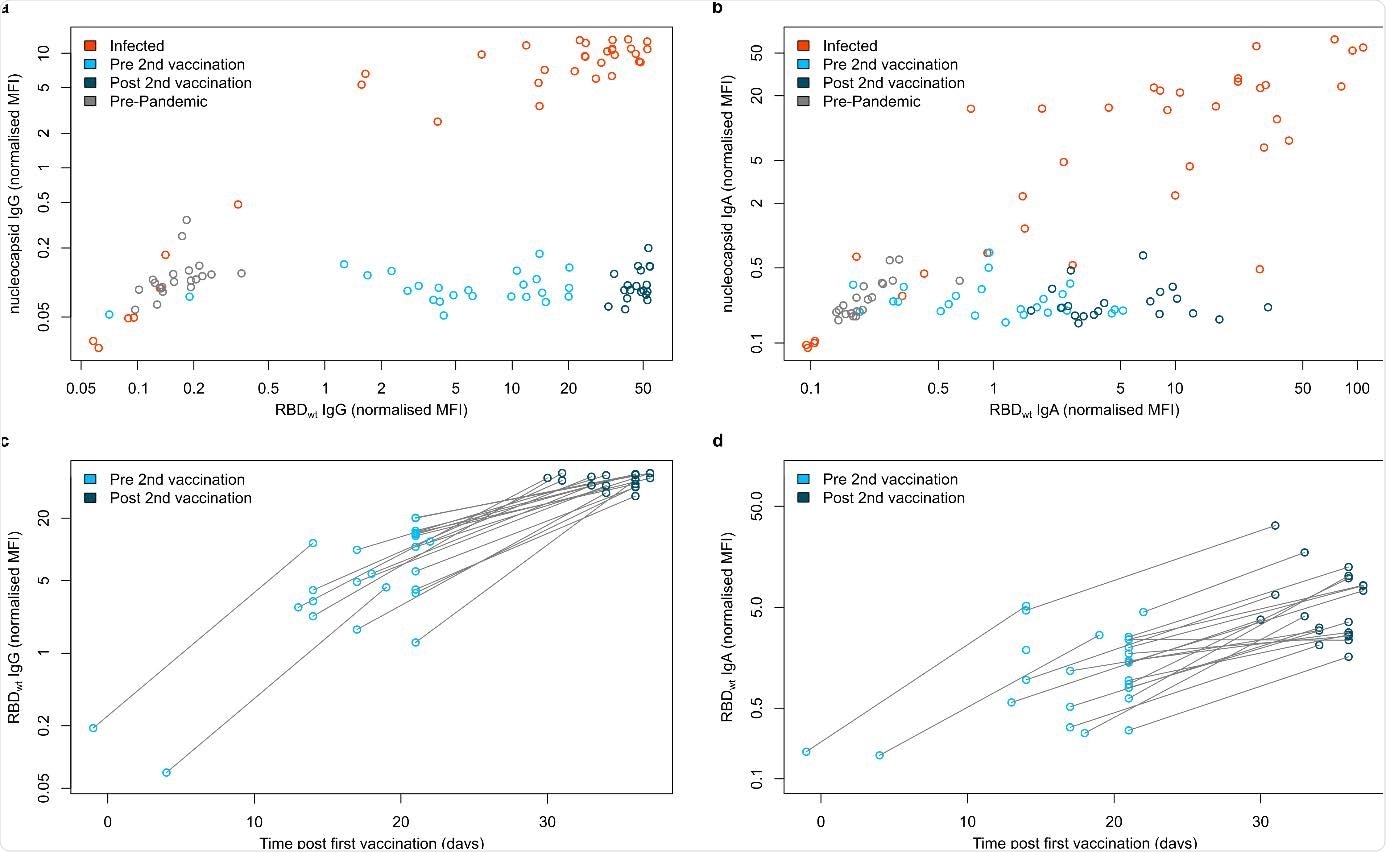
IgG and IgA response in serum samples of vaccinated, infected and negative individuals.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Antibody responses reveal significant variations in RBD mutant binding in new SARS-CoV-2 variants
The researchers found that humoral responses in vaccinated subjects were potent after the second dose of the vaccine. Immunoglobulin G (IgG) antibodies were detected in large amounts in the saliva of these vaccinated individuals. Antibody responses showed significant variations in RBD mutant binding in newly emerging SARS-CoV-2 variants. A considerable reduction in RBD binding and neutralization was observed for the South African variant.
“While only minor differences were detectable for the UK, Mink and LA variants, a substantial reduction in RBD binding antibodies was observed for the South African variant.”
Overall, the study results reinforce the significance of administering the second dose of Pfizer BNT-162b2 vaccine to acquire high levels of neutralizing antibodies. High antibody titers in the saliva indicate that vaccinated individuals may have decreased transmission potential. Significantly reduced neutralization capacity for the South African variant emphasizes the importance of surveillance strategies that help detect new variants and the need for targeting these variants while developing future vaccines.
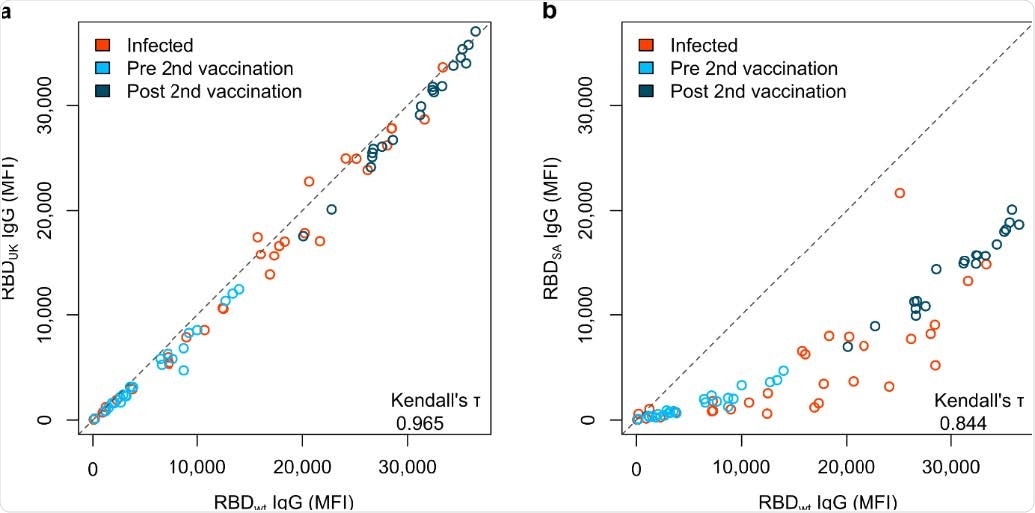
South African RBD mutant has a reduced response compared to UK RBD mutant
Reduced RBD-binding ability to mutants in newer variants highlights the significance of updating current vaccines
According to the authors, future research should focus on the antibody response from different vaccines such as AstraZeneca-Oxford and Moderna vaccines and their persistence against existing or newly emerging RBD mutants over a more extended period in a bigger sample size. In a larger context, the impaired RBD-binding ability to mutants in newer variants of concern shows the significance of updating currently available vaccines accordingly. The South African variant deserves particular attention due to the decreased neutralizing potency identified in the study.
“Viewed in a larger context, the impaired RBD-binding capacity to mutations in emerging variants of concern highlights the importance of updating current vaccines accordingly.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Immune response to SARS-CoV-2 variants of concern in vaccinated individuals, Matthias Becker, Alex Dulovic, Daniel Junker, Natalia Ruetalo, Philipp Kaiser, Yudi Pinilla, Constanze Heinzel, Julia Haering, Bjoern Traenkle, Teresa Wagner, Mirjam Layer, Martin Mehrlaender, Valbona Mirakaj, Jana Held, Hannes Planatscher, Katja Schenke-Layland, Gerard Krause, Monika Strengert, Tamam Bakchoul, Karina Althaus, Rolf Fendel, Andrea Kreidenweiss, Michael Koeppen, Ulrich Rothbauer, Michael Schindler, Nicole Schneiderhan-Marra, medRxiv, 2021.03.08.21252958; doi: https://doi.org/10.1101/2021.03.08.21252958, https://www.medrxiv.org/content/10.1101/2021.03.08.21252958v1
- Peer reviewed and published scientific report.
Becker, Matthias, Alex Dulovic, Daniel Junker, Natalia Ruetalo, Philipp D. Kaiser, Yudi T. Pinilla, Constanze Heinzel, et al. 2021. “Immune Response to SARS-CoV-2 Variants of Concern in Vaccinated Individuals.” Nature Communications 12 (1). https://doi.org/10.1038/s41467-021-23473-6. https://www.nature.com/articles/s41467-021-23473-6.