The main protease of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enzyme implicated in the transcription and replication of the coronavirus. New research led by S. Samar Hasnain from the University of Liverpool in the United Kingdom used the selenium-containing drug Ebselen to analyze the SARS-CoV-2 main protease (Mpro).
They found the selenium atom inactivated cysteine in the main protease's catalytic pocket — preventing SARS-CoV-2 growth and viral replication. The study findings may help with developing therapeutics for inhibiting the virus' main protease.
The researchers write:
"Though our study is of clear immediate interest for SARS-CoV-2, it has wider therapeutic applications of organo-selenium compounds by novel chemical mechanism of the selenation of cysteines of proteases in other current zoonotic beta coronaviruses and those that may emerge in the future."
The study "Novel inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives" is available as a preprint on the bioRxiv* server, while the article undergoes peer review.
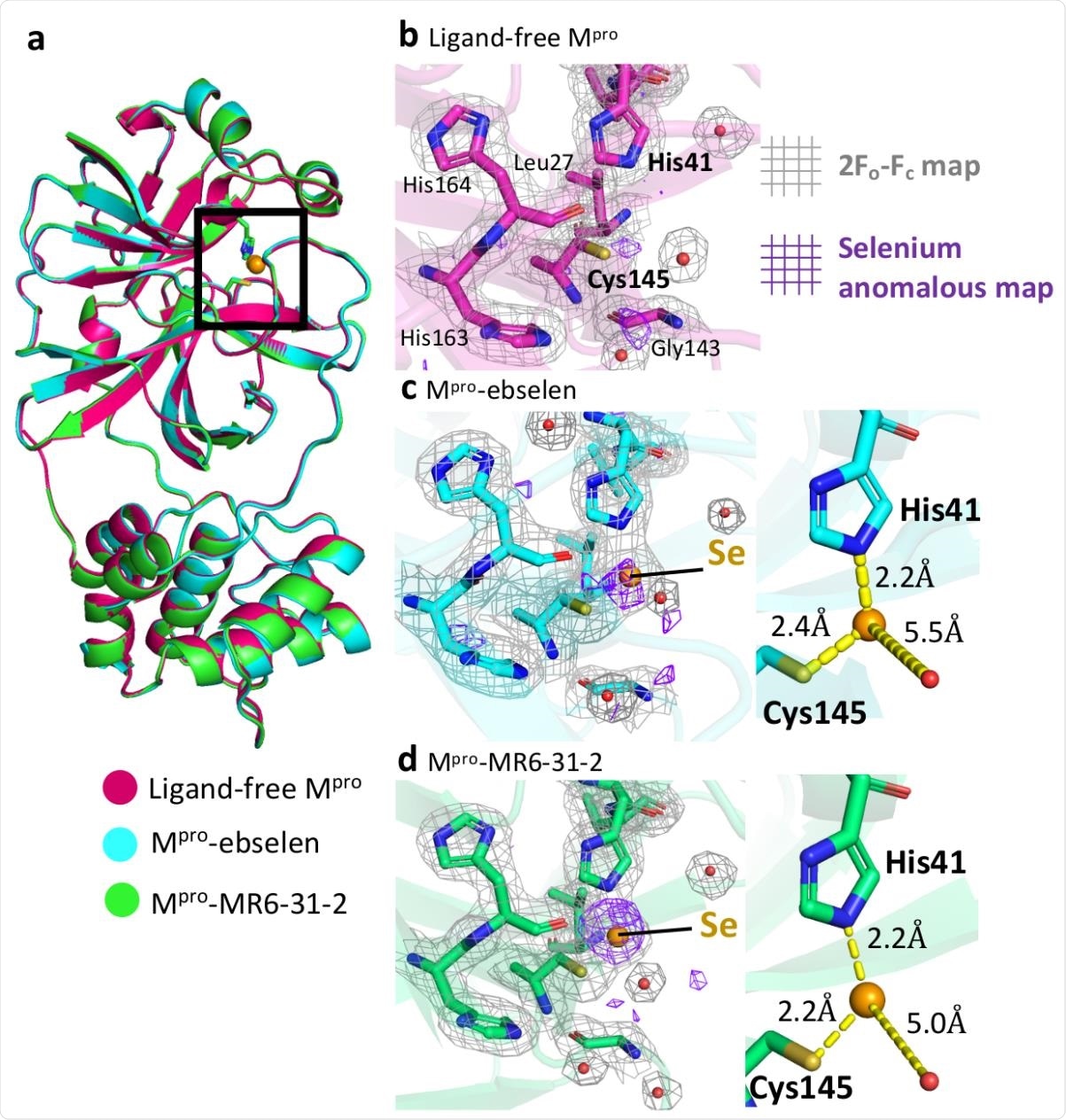
Crystallographic structures of ligand-free Mpro and the complexes with ebselen and MR6-31-2. a, Cartoon representation of superimposed structures of ligand-free Mpro (magenta), Mpro-ebselen (cyan) and Mpro-MR6-31-2 (green). The Mpro catalytic site is highlighted in a black box. Close-up views of catalytic site of b, ligand-free Mpro, c, Mpro-ebselen, and d, Mpro-MR6-31-2. Electron density (2Fo-Fc) map is shown as grey mesh at 1. Anomalous signal of selenium is shown as purple mesh at 3. Selenium atom and waters are shown as orange and red spheres, respectively.
Rationale for the current study

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Previous work from the researchers had used ebselen and five other derivatives to inhibit the main protease of SARS-CoV-2 and block replication. They had found selenium-based ebselen compounds were the most effective in inhibiting the enzyme and viral replication.
The inhibition occurred because ebselen donated a selenium atom and formed a covalent bond at the main protease catalytic site. This caused a blockage of the histidine-cysteine catalytic dyad.
Based on the results, the team hypothesized in this current study that improving selenium-based ebselen using the ebselen scaffold could further enhance antiviral activity.
Results
The researchers investigated ebselen and derivatives' reactivity with Cys145 and whether it could inhibit the proteolytic activity of SAR-CoV-2 main protease.
They found ebselen and the other compounds were potent inhibitors with sub-micromolar levels of IC50. Two derivates of ebselen called MR6-7-2 and MR6-18-2 were twice as effective in inhibiting the main protease.
The team also tested ebselen and derivatives and their ability to stop viral replication in SARS-CoV-2 infected human Vero cells. Mr6-31-2 was three times more effective than the parent ebselen in promoting antiviral activity.
"These results indicate clear on-target interaction of these compounds with Mpro with significant inhibitory power for SARS-CoV-2 and as such potential for development as treatments for COVID-19 patients."
To determine the inhibition mechanism, the researchers used an electron density map of selenium based on diffraction data using X-rays at the wavelength near the selenium absorption edge.
While no selenium density was found in the ligand-free enzyme, there was a strong density in the cysteine-histidine catalytic dyad in the ligand-treated main proteases' structures.
About 60% of selenium occupied the space when using Mpro-ebselen and 80% when using Mpro-MR6-31-2 crystals, consistent with previous data finding binding at the catalytic pocket causing a formation of a selenyl-sulfide bond with Cys145.
The research team observed that the inhibitory activity from ebselen and its derivatives were induced by a selenium atom bound to the main protease catalytic site that inactivated cysteine.
Selenation of the catalytic site occurs because of hydrolysis
Based on the results, the team suggested this mechanism occurs because of hydrolysis at the main protease active site, which releases the phenolic by-product salicylanilide when interacting with ebselen.
The team optimized their LC/MRM-MS method using standard salicylanilide to obtain a chromatogram to confirm this hypothesis. Ebselen samples under incubation showed peaks of 6.37 minutes corresponding to the standard salicylanilide.
The measured concentration of salicylanilide in ebselen samples was 1.28 ng/ml after 4 hours. This indicates the formation of salicylanilide in the incubation of the main protease with ebselen occurs in a time-dependent manner.
The results confirm the mechanism for selenation at the main protease active site occurred due to a hydrolysis product.
"We propose that His41 can assist a water-mediated attack on intermediate adduct 2 in an SNAr type hydrolysis reaction with intermediate 3 possibly stabilized in a manner akin to peptide hydrolysis tetrahedral intermediates within the oxyanion hole of the active site."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Amporndanai K, et al. Novel inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.03.11.434764, https://www.biorxiv.org/content/10.1101/2021.03.11.434764v1
- Peer reviewed and published scientific report.
Amporndanai, Kangsa, Xiaoli Meng, Weijuan Shang, Zhenmig Jin, Michael Rogers, Yao Zhao, Zihe Rao, et al. 2021. “Inhibition Mechanism of SARS-CoV-2 Main Protease by Ebselen and Its Derivatives.” Nature Communications 12 (1). https://doi.org/10.1038/s41467-021-23313-7. https://www.nature.com/articles/s41467-021-23313-7.