Researchers in the United States have developed a novel protein that prevented lethal disease among mice infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19).
The team engineered a soluble, short, and dimeric version of the native host cell receptor that is bound by a surface structure on SARS-CoV-2 called spike during the initial stage of the infection process.
The team – from the Feinberg School of Medicine in Chicago, the University of Chicago, and Northwestern University in Evanston – suspected that a soluble, truncated version of this membrane-bound receptor – called angiotensin-converting enzyme 2 (ACE2) – would serve as a decoy for SARS-CoV-2 spike binding and potentially neutralize infection.
Now, Daniel Batlle and colleagues report that when the novel ACE2 protein was administered in a mouse model rendered susceptible to lethal SARS-CoV-2 infection, the animals only developed mild-to-moderate disease for a few days. Lung pathology had significantly improved in these mice by day 6.
Untreated animals serving as a control group, on the other hand, became severely ill and had to be sacrificed by day 6 or 7.
“Here we report that administration of a novel soluble ACE2 protein to these mice prevented the lethality observed in untreated animals altogether and resulted in a mild disease with reversible lung damage,” says Batlle and the team.
“To our knowledge, this is the first time that a protein that presumably uses the decoy mechanism to neutralize a virus is fully effective in vivo,” they add.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
In vivo studies of viral neutralization by ACE2 have been lacking
The SARS-CoV-2 virus uses full-length, membrane-bound ACE2 as the initial host cell receptor preceding viral entry.
Since ACE2 was identified as the receptor for SARS-CoV-1 as far back as 2003, in vitro experiments have shown that native soluble ACE2 can neutralize the viral spike protein.
“In keeping with this concept early in 2020, we hypothesized that soluble ACE2, by acting as a decoy, could be used as a potential therapeutic approach for SARS-CoV-2 infection,” writes Batlle and colleagues.
“There have been recent in vitro studies testing decoys for SARS-CoV-2,” says the team. “In vivo studies, however, in suitable animal models, to our knowledge, are lacking.”
Batlle and colleagues have previously engineered soluble ACE2 variants that are shorter than native soluble ACE2 and therefore more suitable for treating kidney disease, since they are amenable to glomerular filtration.
One short ACE2 variant comprising 618 amino acids (ACE2 1-618) exhibited high enzymatic activity. When the team fused this variant with a small (5-kD) albumin-binding domain (ABD), the protein’s duration of action in vivo was extended to around 3 days, compared with around 8 hours for naked ACE2 1-618.
Increasing the protein’s binding affinity
The team recently reported that ACE2 1–618-ABD could neutralize SARS-CoV-2 infectivity in human kidney organoids. However, the protein construct is a monomer and the team suspected that viral spike trimers might be more suited to binding dimeric forms of ACE2.
To accomplish dimerization, the team fused ACE2 1-618-ABD with a hinge-like region termed DDC.
In a binding affinity assay, the resulting ACE2 1-618-DDC-ABD construct exhibited an almost 30-fold greater affinity for the receptor-binding domain (RBD) of SARS-CoV-2 than the monomeric ACE2 1-618-ABD.
Next, the team used a transgenic mouse model rendered susceptible to lethal SARS-CoV-2 infection to evaluate the preclinical preventative and therapeutic value of the novel protein in COVID-19.
The mortality rate among these human ACE2-expressing mice (k18-hACE2 mice) is essentially 100% once they are infected with a high viral dose of SARS-CoV-2.
What were the results?
The SARS-CoV-2 infected mice that were treated with ACE2 1-618-DDC-ABD developed mild-to-moderate disease for the first few days.
However, by day 6, lung histopathology had improved and viral titers were markedly reduced in these animals.
“In this model, the efficacy of a novel soluble human ACE2 protein was demonstrated by reducing mortality from 100% to 0% in both male and female animals at day 6 post-viral inoculation,” says Batlle and colleagues.
On the other hand, the untreated control animals developed severe illness and had to be sacrificed by day 6 or 7. Lung histology revealed extensive alveolar hemorrhage and mononuclear and neutrophil infiltrates.
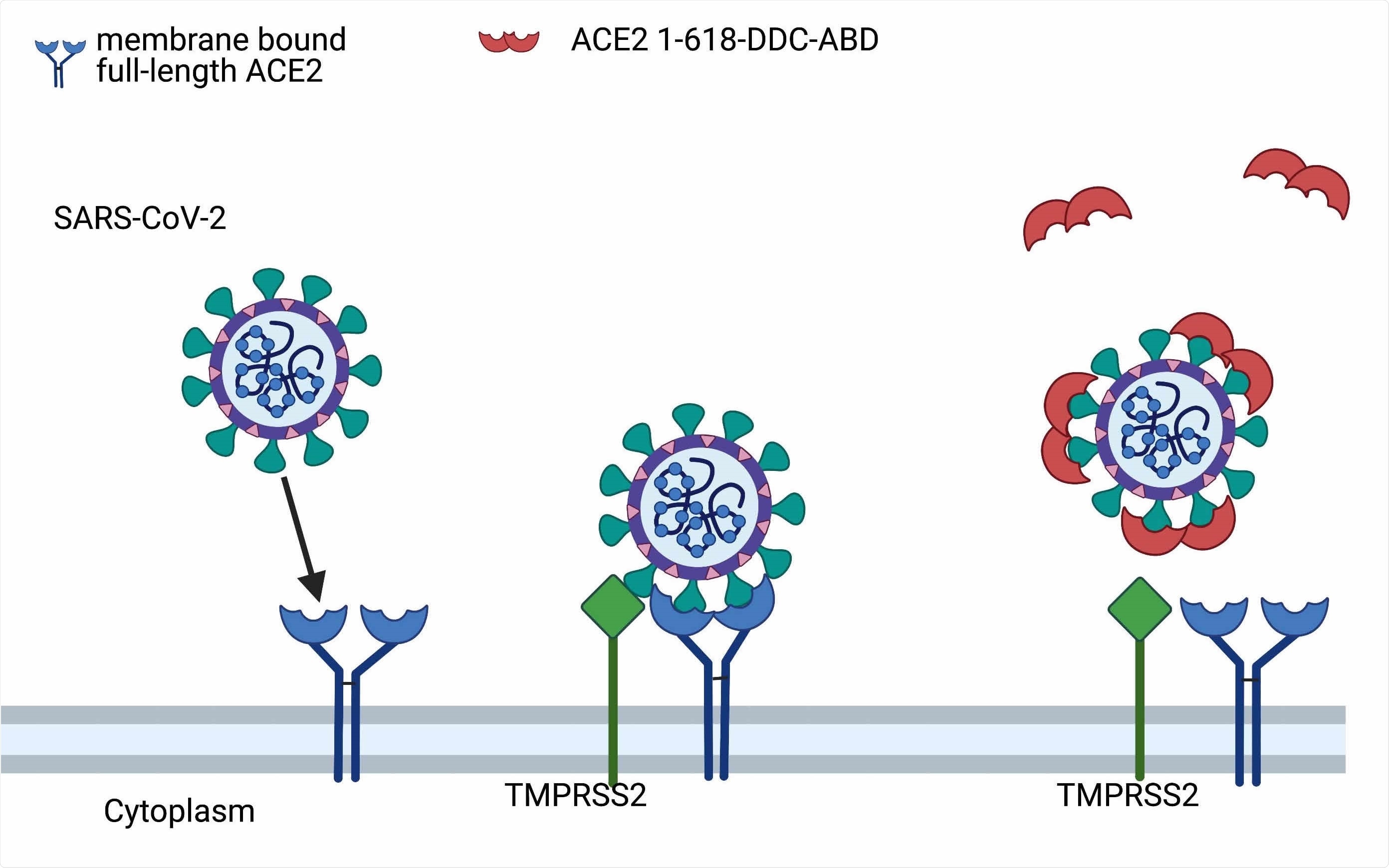
Postulated mechanism of action of ACE2 1-618-DDC-ABD. Administered ACE2 1-618-DDC-ABD (red semi-circles) binds to SARS-CoV-2 acting as a decoy to prevent the binding of SARS-CoV-2 to membrane bound full-length ACE2 receptors (blue). This prevents the internalization of the ACE2-SARS-CoV-2 complex activated by TMPRSS2 (green). Modified from Davidson et al, Hypertension 2020 (10). Created with biorender.com.
Similar approaches may help combat present and future coronavirus outbreaks
The researchers say the findings demonstrate for the first time the complete efficacy of a soluble ACE2 protein for the prevention and treatment of SARS-CoV-2-induced disease in a preclinical animal model.
“Strategies using the same approach are quite likely to be effective in the prevention and treatment of COVID-19 and become an essential complement to combat the present pandemic and future outbreaks of other coronaviruses that use ACE2 as its main receptor,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Batlle D, et al. A novel soluble ACE2 protein totally protects from lethal disease caused by SARS-CoV-2 infection. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.03.12.435191, https://www.biorxiv.org/content/10.1101/2021.03.12.435191v2
- Peer reviewed and published scientific report.
Hassler, Luise, Jan Wysocki, Ian Gelarden, Isha Sharma, Anastasia Tomatsidou, Minghao Ye, Haley Gula, et al. 2022. “A Novel Soluble ACE2 Protein Provides Lung and Kidney Protection in Mice Susceptible to Lethal SARS-CoV-2 Infection.” Journal of the American Society of Nephrology, March, ASN.2021091209. https://doi.org/10.1681/asn.2021091209. https://journals.lww.com/jasn/Fulltext/2022/07000/A_Novel_Soluble_ACE2_Protein_Provides_Lung_and.16.aspx.