The coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), affects many organs in the body. Apart from being a respiratory illness, it can also affect the cardiovascular and digestive systems.
Researchers at the São Paulo State University (UNESP), Sao Jose do Rio Preto, and the Ribeirão Preto Medical School, University of São Paulo (USP) in Brazil, recommend that modulating the gut microbiota and re-establishment of eubiosis amid COVID-19 could help prevent serious complications.
In the paper published in the journal Frontiers in Immunology, the researchers noted that since both the respiratory tract and the gastrointestinal mucosa are affected in COVID-19, it is possible that adjunctive therapies that modulate the gut-lung axis.
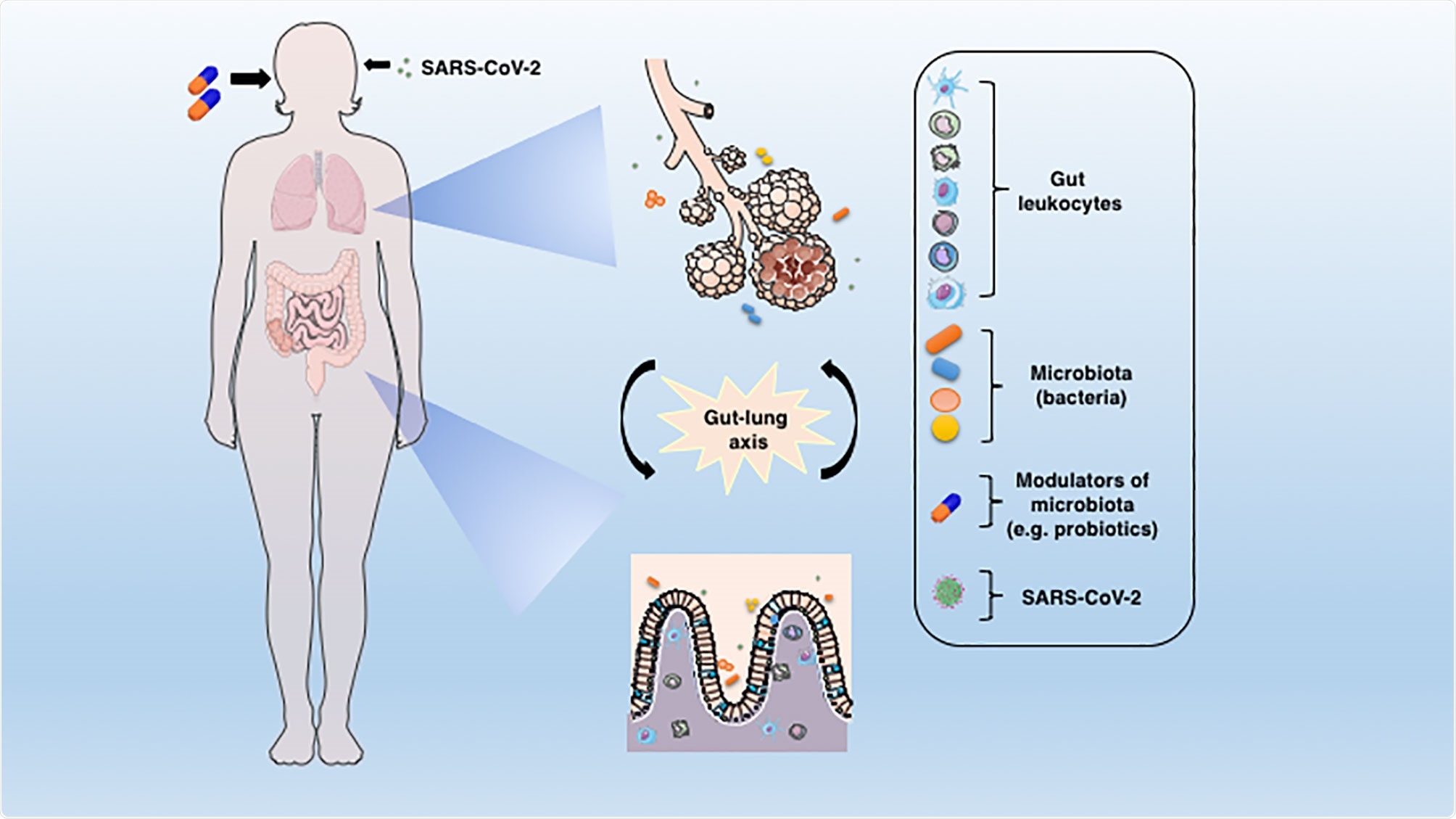
The connection between lung and gut mucosa in the pathogenesis of COVID-19. The SARS-CoV-2 virus infects preferentially cells from the respiratory system, but a large body of evidence points to the GIT as another important target for the virus entry and replication. The dysbiosis, together with the barrier damage and the resulting inflammation may facilitate the disease establishment. The translocated bacteria, leukocytes and the release of inflammatory mediators in the gut-lung axis may contribute to the COVID-19-associated organ deterioration. Some proposed adjunctive therapies such as prebiotics or probiotics, which are aimed at re-establishing the eubiosis state through modulation of microbiota could represent an alternative approach to ameliorate or avoid the worst outcomes of COVID-19.
The gut microbiome
There are trillions of microbes living in the digestive tract. More than 400 species of bacteria thrive in the gut, which is more than the body's cells. These microbes are essential in digesting food, fighting off harmful pathogenic microorganisms, and synthesizing vitamins.
When the equilibrium in the gut microbiome occurs, dysbiosis occurs. It is a condition when the gut's bacteria become imbalanced, causing a wide range of digestive disturbances.
The mucosal surfaces in the lungs and gut play a pivotal role in modulating immune responses by fighting off pathogens and preventing excessive inflammation or tissue damage.
However, this ability depends on the balance of the local microbiota, while a breakdown in the mucosal layer and dysbiosis may favor the establishment of infections, including COVID-19.
Also, there is evidence about crosstalk between the respiratory system and the gastrointestinal tract (GIT), particularly between the intestinal microbiota and the lungs. The interactions and the gut-lung axis have been extensively studied in the past.
The study
The study highlights how SARS-CoV-2 targets the gastrointestinal tract. When the COVID-19 first emerged in December 2019, clinicians first tagged it as a respiratory illness. As the pandemic evolves, more organs and tracts have been affected, including the GI tract.
Many types of viruses such as rotavirus, norovirus, and coronavirus can infect the enterocytes in the GI tract. As a result, there is an impairment in the absorption process, causing an imbalance in intestinal function.
Many reports have shown that SARS-CoV-2 can be detected in feces. In a Singapore study, 50 percent of patients infected with SARS-CoV-2 had the virus detected in their feces.
Another study showed that the presence of SARS-CoV-2 could be seen in both throat swabs and fecal samples.
What are the GI symptoms in COVID-19?
In COVID-19, the most common GIT symptoms include nausea, vomiting, loss of appetite, diarrhea, and stomach cramps.
The presence of GIT symptoms in COVID-19 is common. In China, where the virus first emerged, it was observed in Zheijiang province that among 651 patients with a confirmed diagnosis of COVID-19, between January and February 2020, 11.4 percent had at least one GIT symptom with diarrhea as the most common.
Though COVID-19 is less frequent in children, the prevalence of GIT manifestations was very similar to those of adults. Patients GIT symptoms have a higher rate of chronic liver disease than patients with COVID-19 but without GIT symptoms.
If COVID-19 worsens, GIT symptoms become more prominent. This may be because a viral replication in the GIT may lead to a more serious clinical condition.
Dysbiosis and COVID-19
The gut-lung axis has long been studied. Currently, the study tackled how the microbiota can be affected by SARS-CoV-2 infection. The effect of the intestinal microbiota on systemic immunity and respiratory infections has been explored in both animals and humans.
Some studies have shown the vital role of the microbiota in the lungs' antiviral responses by modulating the immune responses.
The gut microbiome may play a role in expressing type I interferon receptors in respiratory epithelial cells, which respond promptly to viral infections.
Overall, the study researchers recommend experimental therapies based on microbiota modulation, which can help in the fight against the coronavirus pandemic. Several studies have evaluated probiotic and prebiotic administration's effect in reducing the incidence, duration, and severity of respiratory infections in humans.
Experimental studies and clinical trials support the potential for probiotic use during infections with influenza virus, respiratory syncytial virus, and rhinovirus.
The team believes that adjunctive treatments based on the gut-lung axis modulation and re-establishment of eubiosis or a balanced gut microbiome could be an essential therapeutic approach for reducing the severe complications COVID-19 disease.