Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread worldwide, infecting more than 121 million individuals and causing more than 2.68 million deaths since its emergence in December 2019.
The SARS-CoV-2 pathogen can also jump from humans to animals, warranting the need for preventive measures to prevent outbreaks in livestock farms.
Researchers at the University of Wisconsin–Madison aimed to better understand how SARS-CoV-2 interacts with various mammalian angiotensin-converting enzyme 2 (ACE2) receptors. This way, they can identify the factors that influence intra- and cross-species virus transmission.
The study, which appeared on the pre-print server bioRxiv*, showed how mutations in human and other mammalian ACE2s affect spike protein binding using a yeast surface display-based screening method. It can help in the development of effective new vaccines for both humans and livestock animals.
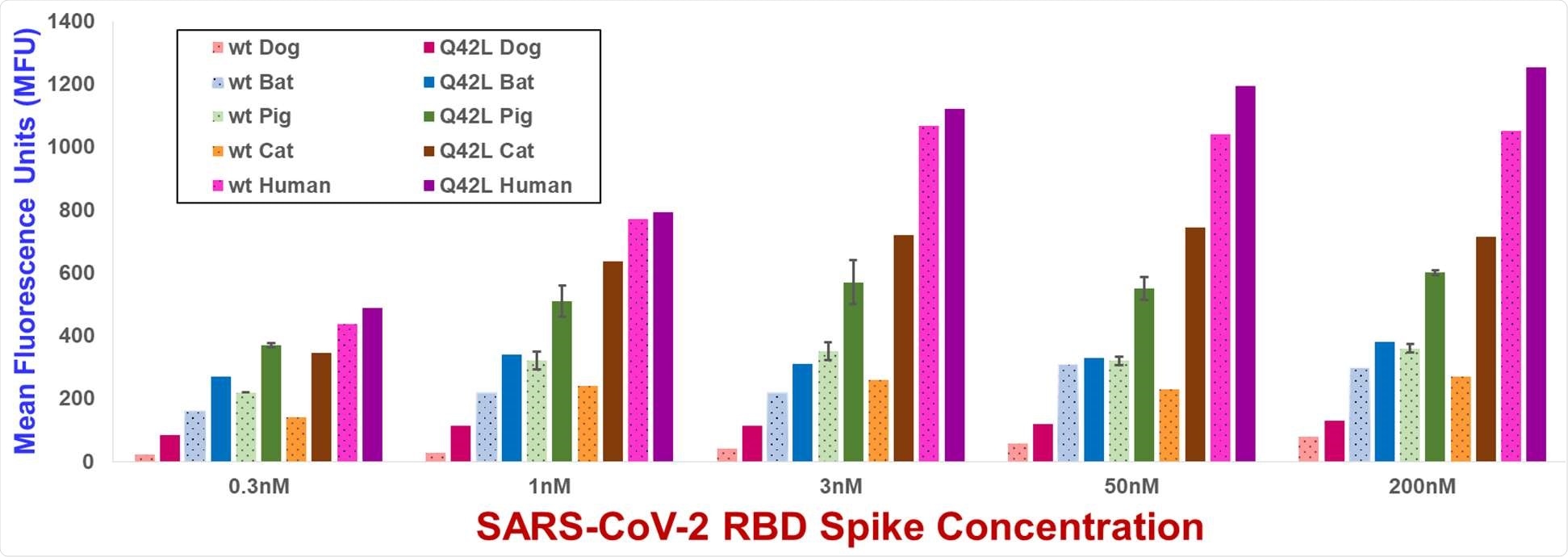
Yeast display flow cytometry spike binding assay MFU values for wild type and Gln42Leu mutant ACE2s. MFU values are the mean of duplicate measurements. Error bars, shown for wild type and Gln42Leu mutant pig ACE2s, represent standard deviations. MFU values for yeast not incubated with spike are typically 30-50 MFU.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
SARS-CoV-2 in humans and animals
SARS-CoV-2 can transfer from animals to humans and vice versa. Over the past year that the pandemic ravaged across the globe, many reports of COVID-19 infections in animals were reported.
Aside from that, SARS-CoV-2 has exhibited a substantial ability to mutate to increase its infectivity and transmissibility in humans. These mutations can potentially reduce the efficacy of existing SARS-CoV-2 vaccines.
It is, therefore crucial to understand how sequence variation in both the ACE2 receptor and the viral spike protein influences transmission within the human population and across species barriers.
The study
The researchers studied how mutations in humans and other mammal ACE2 can impact spike protein binding to arrive at the study findings. They found that ACE2 substitutions at ACE2 amino acid positions 34, 42, and 79 elevated spike binding in various ACE2 orthologs.
Contacts between SARS-CoV-2 spike residues and key ACE2 amino acids identified during ACE2 mutant library screening. (A) ACE2 His34 contacts spike Leu455 and Gln493. (B) ACE2 Gln42 contacts spike Gly446. (C) ACE2 Leu79 contacts spike Phe486. ACE2 residues in green or blue (contact residues). Spike residues in red or magenta (contact residues). Figures generated from Protein Data Bank structure 6m0j (human ACE2) using Pymol.
In fact, the strengthened binding of Gln42Leu and Leu79Ile mutations should be focused on since Gln is at position 42 in 83 percent of annotated mammalian ACE2s, and Leu appeared at position 79 in 56 percent of these proteins.
The study findings that showed ACE2 mutations have similar impacts upon spike binding across many ACE2 orthologs show that spike protein mutations can also control the ACE2-spike binding interaction in a species-independent way.
Further, the team explained that mutant SARS-CoV-2 or other emergent coronaviruses could feature spike proteins that show cross-species transmission tendencies higher than those for SARS-CoV-2 strains currently studied. The findings are significant in vaccine development, animal farming practices to prevent COVID-19 outbreaks, and novel coronavirus strain prospecting.
The results, combined with the structural analysis of ACE2-spike binding interactions, existing evidence on SARS-CoV-2 mutations, and ACE2 sequences among mammalian species, can help shed light on the potential of viral emergence and transmission.
This could also help with the implementation of measures to mitigate the economic and social effects of the current and future coronavirus outbreaks.
“The findings presented in this work, as well as other related computational and experimental pursuits, will contribute to our understanding of how ACE2 and spike RBD amino acid sequences influence SARS-CoV-2 transmission among humans and other mammals. This heightened understanding could play an important role in developing novel vaccines for both humans and animals and contribute to global food security by facilitating the establishment of effective practices for ensuring the health of farmed animal populations,” the authors concluded.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references: