The coronavirus disease (COVID-19) first emerged in late December 2019 in Wuhan, China. Though it has been more than one year since the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported, it is still unclear how long the antibodies persist in those who have recovered.
Researchers at Sinopharm Wuhan Plasma-derived Biotherapies Co., Ltd and Wuhan Institute of Biological Products Co. Ltd, showed a positive rate of immunoglobulin G (IgG) antibody against the receptor-binding domain of the spike protein (RBD-IgG) to SARS-CoV-2 in COVID-19 convalescent plasma donors exceeded 70 percent for 12 months after infection.
The team also noted in the study, which appeared on the pre-print server bioRxiv*, that the RBD-IgG kinetics displayed a downward trend, with the titer starting to stabilize after nine months and dropped by 68.1 percent compared with the first month.
Study background
It has been a long-standing question of how long people who recovered from COVID-19 are protected from reinfection. Several SARS-CoV-2 vaccines have been approved worldwide, but it remains unclear how long these vaccines protect against infection.
The durability of the immune response, particularly humoral immune response, induced by SARS-CoV-2 infection is essential to understand the pathogenesis of COVID-19 and predict the longevity of its vaccine protection.
In infected patients with the severe acute respiratory syndrome (SARS), which caused an outbreak in 2002, the specific antibodies against the virus can last for an average of two years, with the positive rate and titer of severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1)-specific neutralizing antibodies markedly reduced at the third year.
Hence, SARS patients may become vulnerable to the same virus three years after recovering from the infection. This highlights the importance of assessing the humoral immune response's durability to the current spreading virus, the SARS-CoV-2.
The study
To arrive at the humoral immune response duration in convalescent COVID-19 patients, the researchers performed a 12-month longitudinal study by collecting 1,782 plasma samples from 869 convalescent plasma donors in Wuhan City, China. The team also tested specific antibody responses.
They found that the positive rate IgG antibody against the receptor-binding domain of spike protein to SARS-CoV-2 in the COVID-19 convalescent plasma donors surpassed 70 percent for 12 months after infection. This means that the RBD-IgG response in more than 70 percent of COVID-19 convalescent patients could last for about a year, showing that vaccination can help limit the virus's spread.
The RBD-IgG titer dropped by 69.86 percent in the first year compared with the first month's titer. The proportion of the plasma donors whose RBD-IgG titers remained above the moderate titer at the later stages after being diagnosed was 27.2 percent.
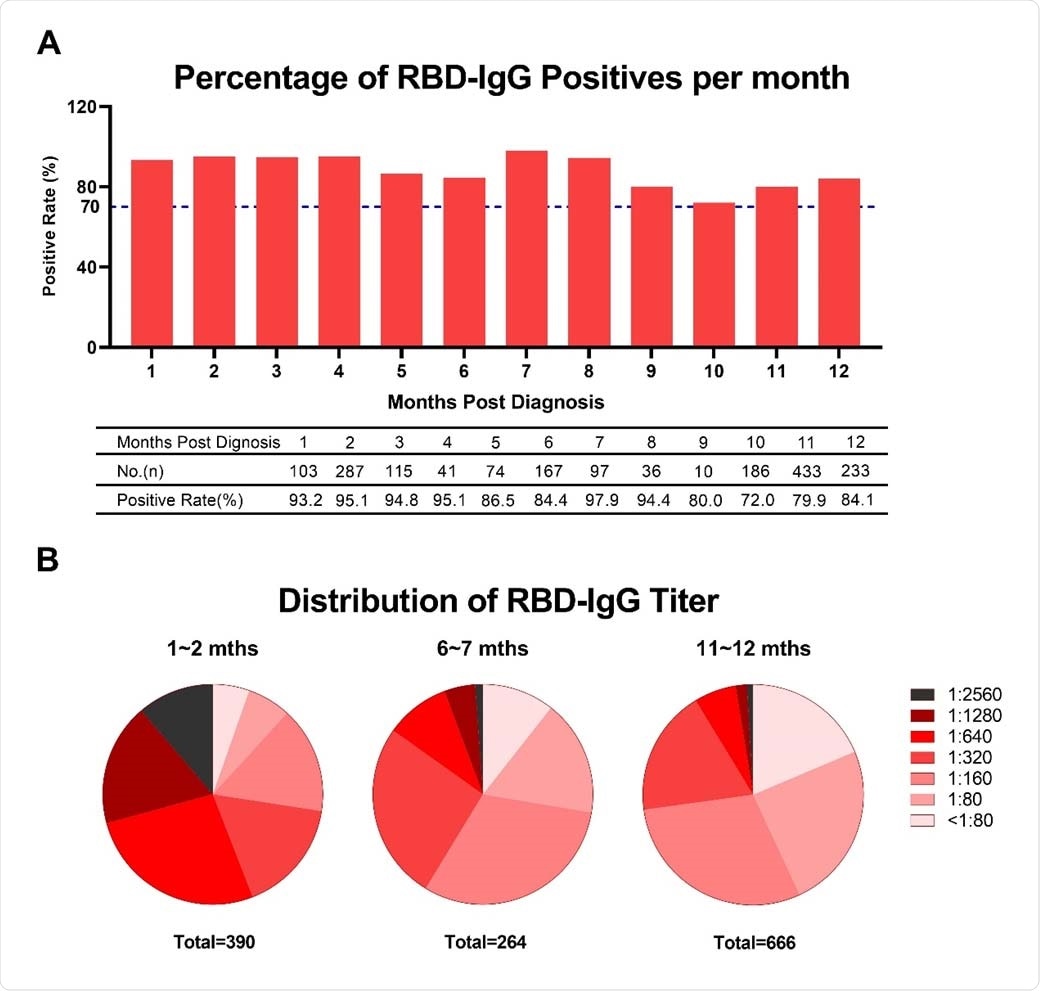
RBD-IgG titers against SARS-CoV-2 over time. (A) Percentage changes of positive RBD-IgG. (B) Changes of RBD-IgG titers distribution. Titers less than 80 were considered as negative.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The team also noted that though the RBD-IgG titer slowly decreased over time within the first year, the RBD-IgG became stable at nine months.
Moreover, the researchers evaluated the RBD-IgG stability with various titers based on the titer value early after diagnosis. Even if more rapid attenuation of RBD-IgG was observed in the plasma donors with elevated titers, after some time, the RBD-IgG in plasma donors with high titers remained higher than those with lower titers.
Consecutively, the team revealed that the RBD-IgG titers markedly increased in 11.67 percent of low-titer patients and 1.87 percent of the moderate-titer population at 10 and 11 months. This could be attributed to the delayed seroconversion in a small number of plasma donors.
The RBD-IgG titers of male plasma donors are higher than those of female plasma donors at the early phase of infection. Meanwhile, the elderly might develop antibody response against SARS-CoV-2, as age is positively correlated with the RBD-IgG titers.
"Furthermore, we confirmed the positive association between RBD-IgG and neutralizing antibody titers," the team note in the study.
"Overall, this study provides strong long-term support for the duration of protection by neutralizing antibodies in COVID-19 plasma donors, indicates the potential to prevent SARS-CoV-2 reinfection, and illustrates the role of neutralizing antibodies in clinical research and development evaluation of vaccines," the team added.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Li, C., Yu, D., Wu, X., Liang, H., Zhou, Z. et al. (2021). Twelve-month specific IgG response to SARS-CoV-2 receptor-binding domain among COVID-19 convalescent plasma donors in Wuhan. bioRxiv. https://www.biorxiv.org/content/10.1101/2021.04.05.437224v1
- Peer reviewed and published scientific report.
Li, Cesheng, Ding Yu, Xiao Wu, Hong Liang, Zhijun Zhou, Yong Xie, Taojing Li, et al. 2021. “Twelve-Month Specific IgG Response to SARS-CoV-2 Receptor-Binding Domain among COVID-19 Convalescent Plasma Donors in Wuhan.” Nature Communications 12 (1). https://doi.org/10.1038/s41467-021-24230-5. https://www.nature.com/articles/s41467-021-24230-5.