Pregnant women are at high risk for severe COVID-19 infection and death. Yet, insufficient safety data on babies has led many guidelines to leave the decision-making and potential threats to future mothers. While the vaccine trials did not directly test pregnant women, more real-world data suggests the vaccine may provide immunity benefits to newborns through breastmilk.
In Israel, there has been an aggressive vaccine distribution campaign with a majority of the population vaccinated. Israel's vaccination efforts have led it to become a case study for vaccine effects on multiple populations.
Now new research led by Michal Kovo from The Hebrew University of Jerusalem suggests fetuses also benefit from vaccination. They found pregnant mothers who developed neutralizing antibodies specific to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) produced from the Pfizer vaccine could be transferred to the fetus.
The researchers write:
"We show herein a robust maternal humoral immune response coupled to a rise in protective antibodies in the fetal circulation as early as 15 days after the first BNT162b2 mRNA vaccination. We further show that mid-pregnancy SARS-Cov2 infection results in prolonged maternal and fetal humoral immunity presented at delivery time."
The study "Maternal to Neonatal transfer of SARS-CoV-2 and BNT162b2 antibodies" is available as a preprint on the medRxiv* server, while the article undergoes peer review.
How they did it
The study took place across 8 Israel hospitals starting in April 2020. Pregnant women needed to be at least 18 years and were being admitted for delivery. Women who actively had COVID-19 illness were excluded.
A total of 1,094 pregnant women were included in the study and divided into three groups. Women with prior COVID-19 infection were in the vaccinated group, women with prior infection were in the unvaccinated group. Pregnant women with no documentation of COVID-19 illness were assigned the control group.
Researchers collected maternal and fetal blood samples along with the umbilical cord from all patients before delivery.
Increased fetal antibody transmission when infected mid-pregnancy
Blood samples showed pregnant women who were positive for SARS-CoV-2 before week 30 had higher transmission levels of IgG specific to the S1, S2, RBD, and N antigens compared to women who were infected after week 30.
Among 65 pregnant women with a history of COVID-19 infection, the top 90% were seropositive with an IgG response for N antigens. In contrast, 14% of the control group were considered seropositive, indicating a preexisting induced immunity due to infection. About 8% of vaccinated women were seropositive for N, of which three had tested positive for SARS-CoV-2.
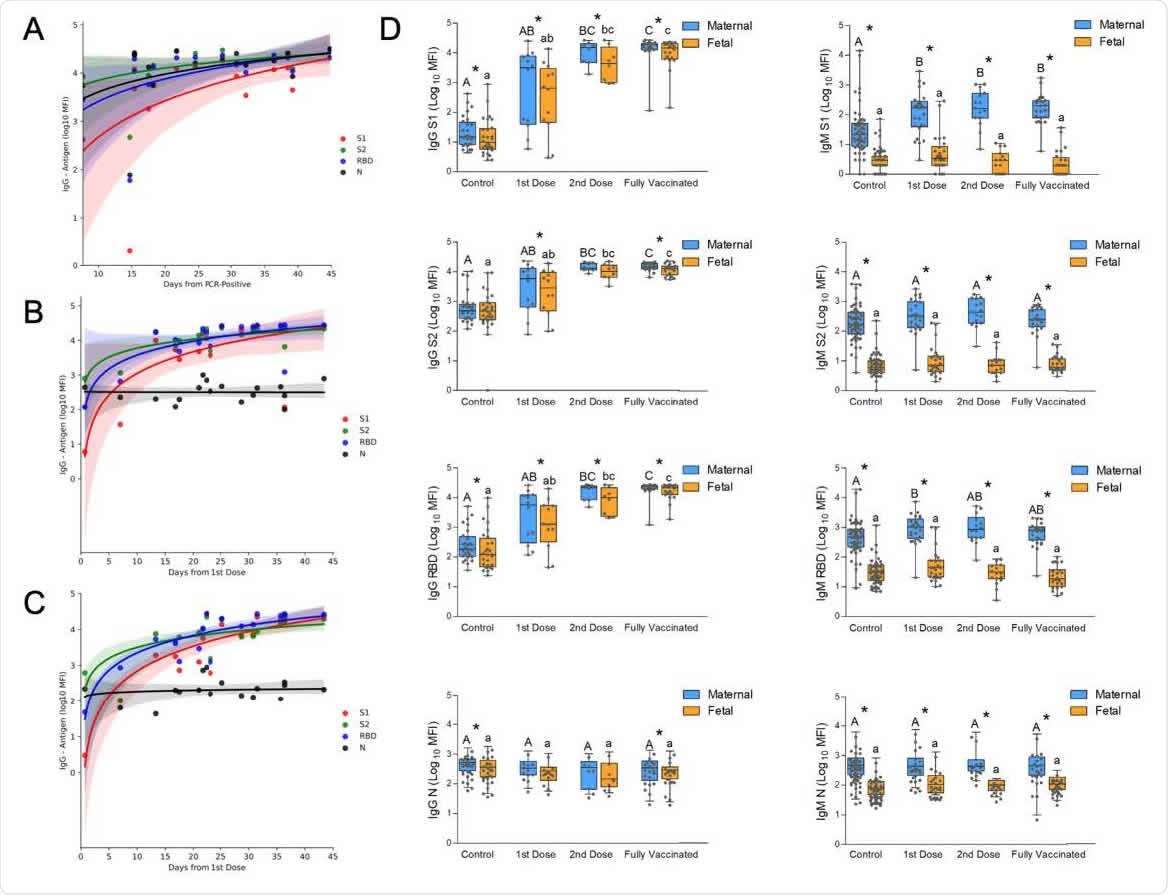
Temporal changes of the acute immune response to SARS-Cov2 infection and to vaccination in pregnancy. (A) Analysis of the change in maternal IgG during the first 50 days after positive RT-PCR, derived from the GA of positivity and the GA of delivery (see the full timeline data for immune response across pregnancy in Figure 1). (B) Analysis of maternal IgG response to BNT162b2 vaccination derived from the GA of the first vaccine and the GA of delivery. A second dose was administered on Day 21. . (C) Analysis of the temporal changes of fetal IgG following BNT162b2 vaccination derived from the GA of the first vaccine and the GA of delivery. A second dose was administered on Day 21; shaded areas in (A), (B) and (C) show the 95% confidence interval (D) Serological data of maternal-fetal pairs was derived from control, unvaccinated serologically negative (N-) mothers; as well as vaccinated mothers grouped for deliveries in the first 3 weeks after the 1st vaccine; deliveries during the first week after the 2nd vaccine; and fully vaccinated who delivered more than a week after the second vaccine. Left columns, IgG; right columns, IgM; from top to bottom, serological response to S1, S2, RBD, and N. Statistical significance: A,B,C above the blue bars indicate significant differences among the groups in maternal antibodies, while a,b,c above the orange bars indicate significant differences among the groups in fetal antibodies (Kruskall–Wallis one-way ANOVA test, following by Dunn's All-Pairwise Comparisons Test); *indicate a significant difference between maternal and fetal antibodies within the same group (Paired test).
Immune response after vaccination compared to natural infection
The researchers observed a steady increase in IgG humoral response specific to S1, S2, receptor-binding domain, and N antigens 45 days after infection.
Vaccinated women developed a faster immune response. They had an elevated IgG response to S1, S2, the receptor-binding domain — but not N antigens — 15 days after receiving their first Pfizer dose. The IgG response further increased after the second Pfizer dose.
Results showed vaccinated pregnant women had significantly higher immune responses toward S1 and the receptor-binding domain of SARS-CoV-2 compared to unvaccinated women at the time of delivery. Although, IgG levels for S2 and N were higher in women who recovered from COVID-19 infection than vaccinated women.
Indeed, the low S2 and N antigens observed in vaccinated women translated to lower IgG responses of the two antigens in fetal blood samples. Fetal IgG levels for S1 and the receptor-binding domain did not differ between vaccinated and unvaccinated women who recovered from natural infection.
Fetal IgG levels towards SARS-CoV-2 antigens
There were significant increases in maternal and fetal IgG and maternal IgM levels to S1, S2, and receptor binding domain but not N after the first Pfizer vaccine dose. The enhanced immunity levels persisted during various time checks in the study. The fetal IgM response was negligible, which the researchers suggest is consistent with the lack of evidence for the fetus's direct exposure to vaccine-derived antigens.
There were significant positive correlations between all fetal and maternal IgG antibodies, suggesting similar placental antibody transfers following vaccination or recovery from natural infection. Significant differences for the maternal to fetal transfer ratio for S1, S2, and the receptor-binding domain were found between unvaccinated and vaccinated pregnant women.
Important Notice
*medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.