Initial clinical trials for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines excluded pregnant women. Thus, pregnant women's immune response to vaccination and the transplacental transfer of maternal antibodies to the fetus is not yet studied in detail.
Recently, a group of researchers analyzed 122 pregnant women and their neonates at the time of birth. All the women studied received 1 or both doses of a messenger RNA (mRNA)-based vaccine for COVID-19. Of the 122 women part of the study, 55 had received only the first dose of the vaccine while 67 women had received both doses of the mRNA vaccine by the time they gave birth; 85 women had received the Pfizer-BioNTech vaccine, and 37 women received the Moderna vaccine. The study is published on the preprint server, bioRxiv*.
All the participants tested negative for SARS-CoV-2 on nasopharyngeal swabs using reverse-transcriptase (RT)-PCR. None of the women reported any symptoms of COVID-19 at the time of admission for delivery. They also tested negative for antibodies against the nucleocapsid protein (NP) antigen, which helps ensure that the detected antibodies were not produced against a past SARS-CoV-2 infection.
The researchers used an ANOVA with Tukey post hoc to analyze the relationship between IgG antibody levels over time and Pearson correlation analysis and linear regression on log2-scaled serological values to study the relationship between maternal and neonatal IgG antibody levels.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Results show that over 98% of neonates born to women who received both vaccine doses had detectable IgG levels
According to the study results, pregnant women who received mRNA-based vaccines for SARS-CoV-2 during their pregnancy had detectable IgG and IgM responses at the time of birth. When tested at birth, 87 women produced only an IgG response, 19 women produced both IgM and IgG response, and 16 women showed no detectable antibody response. All the women were within 4 weeks after their first dose of the vaccine.
Over time, the number of women who had an antibody response and those who passed on passive immunity to their neonates increased. All women studied and their neonates, except for 1 neonate, had detectable levels of IgG antibodies by 4 weeks post maternal dose 1 of the vaccination.
Over 43% (24/55) of neonates born to women who received only 1 dose of the vaccine had detectable IgG, and over 98% (65/67) of neonates born to women who had received both doses of the vaccine had detectable IgG. There was a weekly increase in IgG levels in pregnant women from 2 weeks after the first dose of the vaccine, and between the 1st and 2nd weeks after the second dose of the vaccine. The results also showed that maternal IgG levels were linearly associated with IgG levels in the neonates.
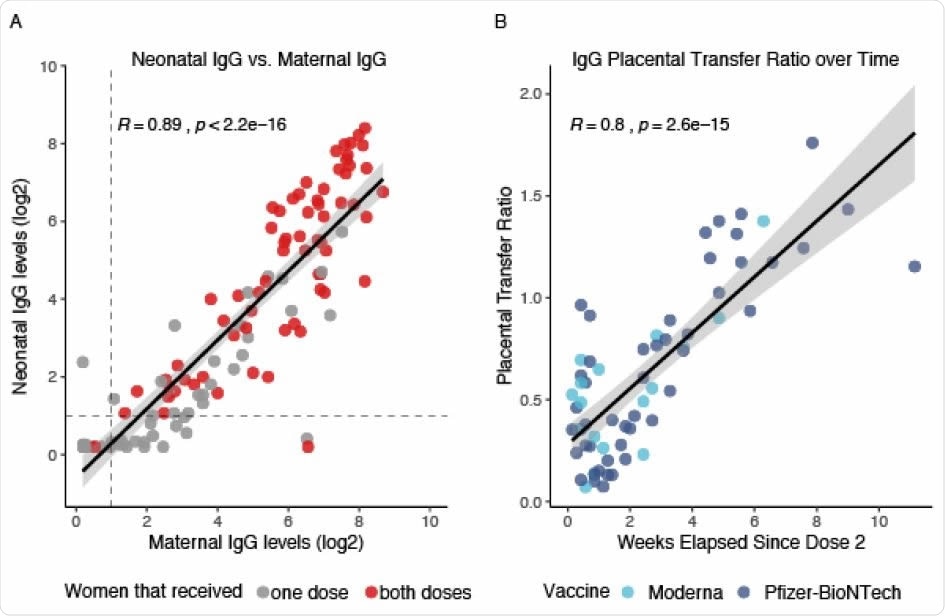
Neonatal Antibody Response to Maternal SARS-CoV-2 mRNA Vaccination. A. Neonatal IgG levels vs. maternal IgG levels. Grey dots represent neonates born to mothers that only received one dose of the vaccine. Red dots represent neonates born to mothers that received both doses of the vaccine. All positive serology cutoffs were 1 (dashed line). B. Placental transfer ratio (Neonate IgG / Maternal IgG) vs. weeks elapsed since maternal vaccination dose 2 for 65 mother-baby dyads containing mothers that received both vaccine doses. Time point 0 is day of vaccine dose 2. Light blue dots represent maternal Moderna vaccination, dark blue dots represent maternal Pfizer-BioNTech vaccination. Women received either the Moderna or the Pfizer-BioNTech vaccines.
Findings suggest that timing between vaccination and birth may be crucial in pregnant women
The study's findings show that, in pregnant women, mRNA-based COVID-19 vaccines induce maternal antibody production by 5 days after the 1st vaccination dose and offer passive immunity to the neonate by 16 days post the first dose of maternal vaccination.
The increase in maternal IgG and the increased placental IgG transfer ratio over time indicate that the timing between vaccination and birth could be a significant factor that should be considered while devising vaccination strategies for pregnant women.
"Further studies are needed to understand the factors that influence the transplacental transfer of IgG antibody, as well as the protective nature of these antibodies."

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Antibody response to SARS-CoV-2 mRNA vaccines in pregnant women and their neonates Malavika Prabhu, Elisabeth A. Murphy, Ashley C. Sukhu, Jim Yee, Sunidhi Singh, Dorothy Eng, Zhen Zhao, Laura E. Riley, Yawei J. Yang bioRxiv 2021.04.05.438524; doi: https://doi.org/10.1101/2021.04.05.438524, https://www.biorxiv.org/content/10.1101/2021.04.05.438524v1
- Peer reviewed and published scientific report.
Prabhu, Malavika, Elisabeth A. Murphy, Ashley C. Sukhu, Jim Yee, Sunidhi Singh, Dorothy Eng, Zhen Zhao, Laura E. Riley, and Yawei J. Yang. 2021. “Antibody Response to Coronavirus Disease 2019 (COVID-19) Messenger RNA Vaccination in Pregnant Women and Transplacental Passage into Cord Blood.” Obstetrics & Gynecology, July, 10.1097/AOG.0000000000004438. https://doi.org/10.1097/AOG.0000000000004438. https://journals.lww.com/greenjournal/Citation/2021/08000/Antibody_Response_to_Coronavirus_Disease_2019.15.aspx.