The coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a respiratory virus with an unpredictable clinical profile. The most severe clinical features occur in elderly and frail individuals, as well as those with certain predisposing medical conditions such as obesity, hypertension, and diabetes.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
High impact of COVID-19 on care homes
The reasons for the high toll in this population include advancing age, frailty, and the presence of comorbid conditions. The high degree of physical contact between the staff and residents of such homes also impacts the virus's rapid spread.
Non-pharmaceutical interventions (NPIs) such as avoiding visitors, restricting inhabitants to private rooms and limiting social interactions are unpleasant but important aspects of limiting the toll of this infection. These measures may have helped control the number of cases in nursing homes.
Vaccination-associated improvement
However, the rollout of vaccines is credited with an 80% reduction in cases, and a 65% decline in deaths, in these centers. This has led to a renewed impetus for calls to relax or abandon existing restrictions on social interactions between nursing home residents, and between them and outside visitors.
While this is beneficial in terms of increased social exposure for the residents, the suggestion has been controversial. The Centers for Medicare and Medicaid Services have issued guidelines for reopening nursing homes. Still, no official guidelines have been released to reopen other assisted living, personal care, or independent living facilities.
Current vaccines have effectively reduced the rates of severe COVID-19, but data on the humoral immune response to the vaccine is still being collected. In response, the Society for Post-Acute and Long-Term Care Medicine (AMDA) has backed a slowly phased reopening of such centers.
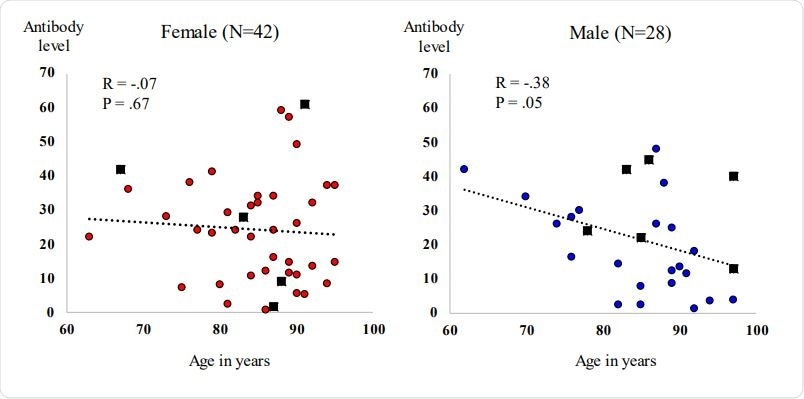
Scatter plot of patient age (x-axis) by Beckman Coulter antibody level (y-axis). Females (left plot) with red-filled dots depicting participants without a prior history of COVID19, and black-filled rectangles depicting participants with a prior history of COVID-19. Males (right plot) with blue-filled dots depicting participants without a prior history of COVID-19, and black-filled rectangles depicting patients with a prior history of COVID-19.
Preliminary study for risk assessment
Few studies have focused on evaluating the level of risk of the residents of such homes following reopening instead of discussing the steps to be taken to complete this process. The current study uses antibody measurements to understand the level of risk of these individuals, especially after vaccination.
Study details
The target population includes older adults living in assisted care, personal care and independent living facilities, with or without a history of natural infection with SARS-CoV-2, and following two doses of a COVID-19 vaccine.
Cancer patients and those on immunosuppressive therapy were excluded from this study. Both total immunoglobulin (Ig) antibodies, and IgG antibodies, to the virus were measured using two independent assays. Both were based on the S1 subunit of the spike antigen.
Of the 70 participants, the age ranged between about 60 and 97 years, but half were aged 80-90 years. A quarter of each were younger and older, respectively. The vast majority were White, and 60% were women.
Most of the participants had taken two doses of the Moderna vaccine (~99%), and 1.4% of the Pfizer vaccine within the 50 days before the study period.
What were the results?
Antibody levels were present in all participants but with wide variations. Older men tended to show lower levels, as did those on steroids. Antibody levels also appeared to be lower with duration since vaccination.
A sixth of the participants had a history of natural infection from which they had recovered. Most of them tended to have higher antibody levels. Still, in one person, the very low antibody levels stood out as proof that having had COVID-19 does not always guarantee higher humoral responses to vaccination.
What are the implications?
The implication is that "moderate to higher functioning adults, even of advanced age, do mount detectable antibody responses when vaccinated with COVID-19 mRNA-based vaccines."
However, there were marked inter-individual differences in the titers measured. This finding suggests that reopening strategies may be proceeded with once the population of such a home has been vaccinated.
It is necessary to point out that this conclusion is based on the supposed correlation of a protective immune response with the presence of anti-SARS-CoV-2 antibodies. Indeed, immunity is a complex phenomenon with both humoral and cellular responses, which are often interdependent and involving both innate and adaptive immunity.
Further study will be essential to understand the type and functional status of the antibodies produced in this population after vaccination, the neutralizing ability of the antibodies, and how long this protective effect, if present, lasts.
"Clinical protection is afforded not just by pre-formed antibody levels, but by ongoing adaptive immunity, which is known to be decreased in older individuals." This demands ongoing clinical monitoring to gather data which must then be built into a continuing risk assessment protocol for each facility.
The small size of the sample also prevents any definitive conclusions from being drawn. Rather, this may be an early feeler about the state of the immune system in this population post-vaccination, especially as frail and immunosuppressed people were unlikely to have been part of this study.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources