Although monoclonal antibodies (mAbs) are an effective treatment option against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for limiting viral transmission and decreasing the load on hospitals, their use is limited, especially in vulnerable populations.
Comparing clinical outcomes in mAb-treated and untreated SARS-CoV-2 patients
Recently, researchers from the U.S. reviewed electronic health records of SARS-CoV-2 patients at a medical center that started mAb infusions in January 2021 with the support of the National Disaster Medical System, part of the U.S. Department of Health and Human Services. The study is published on the preprint server, medRxiv*.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
SARS-CoV-2 patients who received mAb infusions were compared with untreated patients who satisfied the eligibility criteria for mAb treatment. The researchers used logistic regression to assess the effect of mAb treatment on the risk of E.R. visit or hospitalization within 30 days of lab-confirmed COVID-19.
Only 1.9% of mAb-treated patients need a hospital visit within 30 days of testing positive for
SARS-CoV-2
Out of the 598 COVID-19 patients who were part of the study, 270 or 45% of the patients received bamlanivimab, and 328 or 55% of the patients were untreated. Thirty-nine percent or 231 patients were Hispanic. Among patients treated with mAbs, only 5 out of 270 (1.9%) presented to the E.R. or needed hospitalization within 30 days of testing positive for SARS-CoV-2, compared to 39 out of 328 (12%) untreated patients.
The risk of hospitalization or E.R. visit was 82% lower in patients treated with mAbs compared to untreated patients after adjusting for gender, age, and presence of comorbidities.
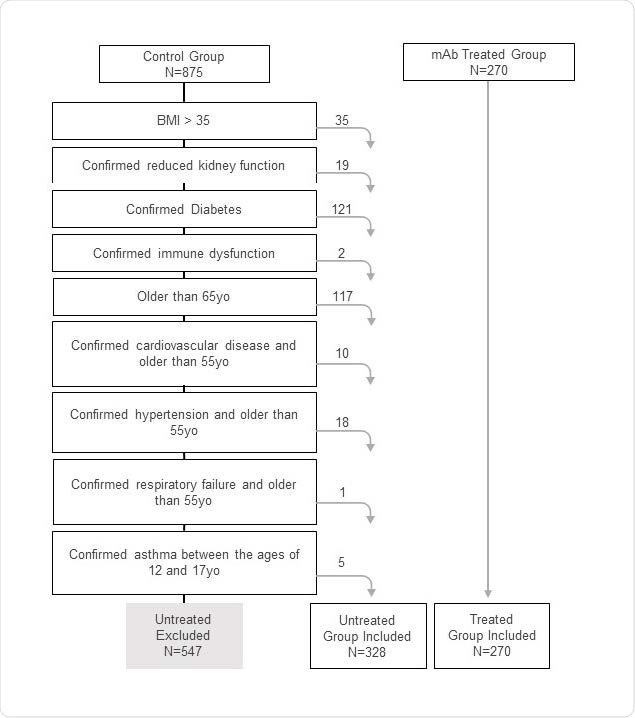
Flow diagram applying the inclusion criteria to collected health records that generated the final study population.
Findings support broader treatment of COVID-19 patients with mAbs to reduce the burden on hospitals
This study was conducted in a diverse, real-world population of COVID-19 patients at risk of progression to severe disease and showed that mAb treatment significantly lowered the risk of subsequent hospitalization or E.R. visit. The results of the study demonstrated that a single infusion of bamlanivimab within 10 days of onset of COVID-19 symptoms considerably decreased the risk of hospitalization related to COVID-19. The association between mAb treatment and better clinical outcomes remained significant after adjusting for factors such as age, race, ethnicity, and pre-existing conditions. BMI > 35 remained strongly associated with disease progression leading to hospital visits after adjusting for mAb treatment.
“A significantly larger proportion of untreated patients had co-morbidities that increase the risk of severe COVID-19 outcomes compared to treated patients in the current study, notably a higher proportion with elevated BMI.”
Individuals who are at increased risk of SARS-CoV-2 infection and those at risk of severe disease are being prioritized for COVID-19 vaccination in the U.S. However, treatment options such as mAb infusions are crucial for those who are unvaccinated due to vaccine hesitancy contraindications. Although studies show that the newer viral variants are poorly neutralized by many mAbs, the researchers believe that minor adjustments to the existing mAb products can help resolve this problem. In addition to this, the FDA has issued some guidance endorsing use of existing mAb formulations and clinical protocols to facilitate rapid review and introduction of these modified mAbs to the general public.
“The continued U.S. government efforts to increase access to mAbs are intended to ensure that COVID-19 therapeutics are equally available to all patients - an important national health equity consideration.”
To summarize, the findings of this real-world study show that mAb treatment with bamlanivimab resulted in a roughly 80% reduction in the risk of hospital visits in COVID-19 patients. Increasing availability and use of new COVID-19 treatment options may help improve patient outcomes, and reduce the burden on the healthcare system, thus contributing to better health equity in the U.S.
Thus, the results support broader treatment with mAbs, especially in disadvantaged populations.
“Our results are consistent with prior clinical trial data showing a 70% reduction in medical visits by mAb-infused patients compared to placebo controls.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Real-world Effect of Monoclonal Antibody Treatment in COVID-19 Patients in a Diverse Population in the United States Kaitlin Rainwater-Lovett, John T. Redd, Miles A. Stewart, Natalia Elías Calles, Tyler Cuff, Mike Fang, Mark J. Panaggio, Anastasia S. Lambrou, Jonathan K. Thornhill, Christopher Bradburne, Samuel Imbriale, Jeffrey D. Freeman, Michael Anderson, Robert Kadlec, medRxiv, 2021.04.08.21254705; doi: https://doi.org/10.1101/2021.04.08.21254705, https://www.medrxiv.org/content/10.1101/2021.04.08.21254705v1
- Peer reviewed and published scientific report.
Rainwater-Lovett, Kaitlin, John T Redd, Miles A Stewart, Natalia Elías Calles, Tyler Cluff, Mike Fang, Mark J Panaggio, et al. 2021. “Real-World Effect of Monoclonal Antibody Treatment in COVID-19 Patients in a Diverse Population in the United States.” Open Forum Infectious Diseases 8 (8). https://doi.org/10.1093/ofid/ofab398. https://academic.oup.com/ofid/article/8/8/ofab398/6327795.