Researchers tested the antibodies elicited from mRNA vaccination and compared them to those from natural SARS-CoV-2 infection. They found the vaccine did not have antibodies to the virus nucleocapsid protein but had potent RBD antibodies.
Several vaccines have been approved for combatting the COVID-19 pandemic. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA)-based vaccines, for example, those developed by Moderna and Pfizer, have shown exceptional efficacy. Evidence suggests strong protection within two weeks of vaccination.
Researchers from the University of California, Irvine, investigated the immune response produced by mRNA vaccines to better understand how they compare to antibodies generated by natural severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Their results are published on the bioRxiv* preprint server.
The authors used the data from ongoing seroprevalence studies in Orange County, California. The first survey was conducted in July 2020, and the second one was done in December 2020. Samples collected from surveys by the University of California Irvine Medical Center in May and December 2020 were also analyzed.
Samples from vaccinated individuals were collected in the months of January, February, and March 2021. They used the coronavirus antigen microarray to measure antibodies against 37 coronaviruses and influenza antigens.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Antibodies different in vaccination and natural infection
The seroprevalence in the Santa Ana zip codes was 18% in July 2020 and 26% in December 2020. At the hospital, the seroprevalence was 13% in December 2020. After vaccination started at the hospital, there was 98.7% seroprevalence by the end of March 2021, suggesting the mRNA vaccine is able to elicit a strong antibody response.
There was a difference between the antibodies elicited by natural infection compared to that from the vaccine. Since the vaccine does not have the nucleocapsid protein, there are no antibodies against this in the vaccine-induced antibodies. However, antibodies against nucleocapsid were seen in natural infection, suggesting this could be a biomarker for natural infection.
Further testing revealed that vaccines elicit more antibodies against the spike protein receptor-binding domain (RBD) compared to the antibodies seen in natural infection. All individuals had antibodies to seasonal flu, and cold and the levels were the same for all irrespective of whether they had COVID-19.
Natural infection produces antibodies to the nucleocapsid and all fragments of the spike protein. The highest antibody levels were against the nucleocapsid, full-length spike protein, and the S2 subunit. Antibody levels against RBD were weak and could be a mechanism for new virus variants to evolve
Vaccinated individuals showed high antibody levels against the full-length spike protein, S2 subunit, and much higher levels to the RBD and S1 subunit. These individuals also had cross-reactive antibodies between the spike protein and RBD, absent in natural infection.
The mRNA vaccine likely adopts a protein conformation that presents cross-reactive epitopes. This could be useful against emerging virus variants and suggests the antibodies produced could still be effective against them.
mRNA vaccines elicit a strong antibody response
Natural infection produces a uniform level of antibodies against the nucleocapsid and spike protein. Vaccinated individuals fall into two groups, those with antibodies against the nucleocapsid protein and those without. Those with nucleocapsid antibodies may have been infected naturally before.
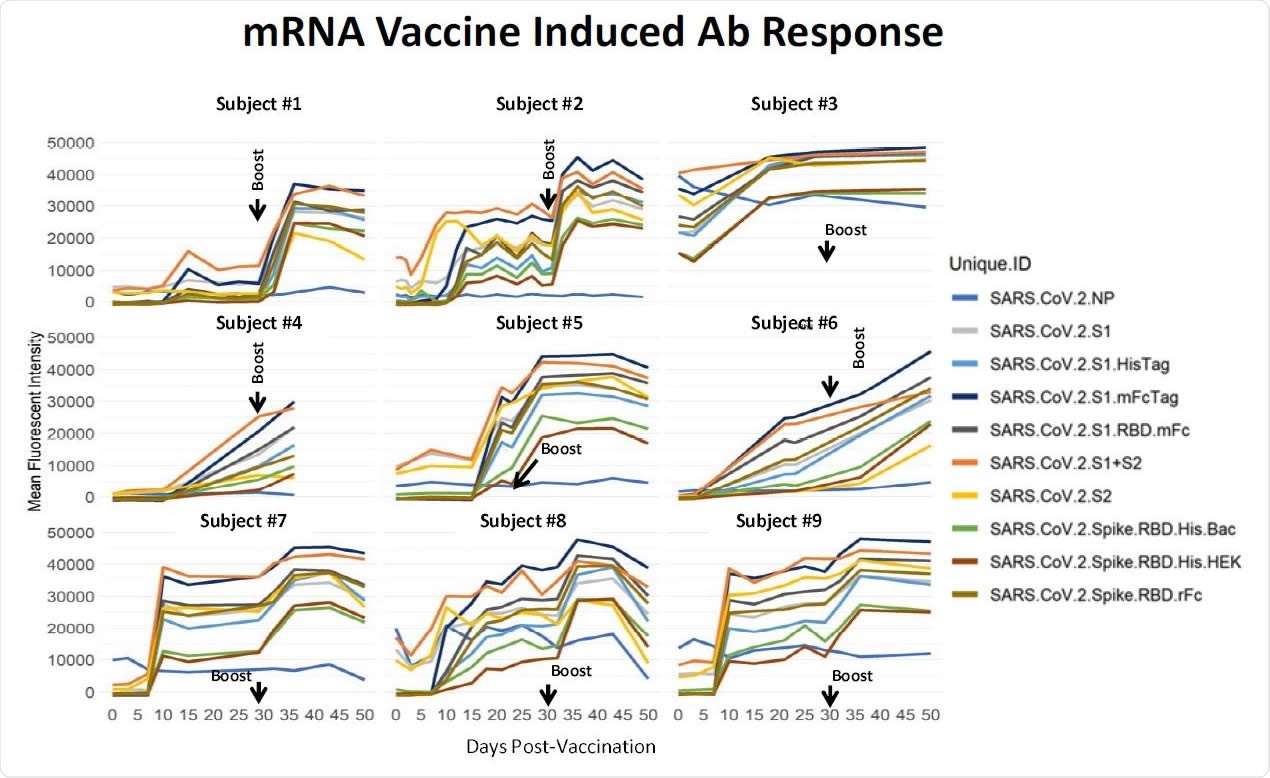
Longitudinal specimens taken at weekly intervals from 9 individuals pre- and post-mRNA vaccination. Individual differ substantially in their response to the prime. Five individuals had low baseline NP reactivity that did not change post-vaccination. Four individuals had elevated NP reactivity at baseline which also did not change significantly post-vaccination; subject #3 was a recovered confirmed COVID case. In this small group, higher baseline NP predicts a higher response after the prime. These results support a directive to get the boost in order to achieve more uniform protection within a population of individuals.
Some individuals showed good antibody levels after the first dose, but most required a booster dose for robust antibody levels, which were seen about 35 days after the first dose. The data also suggests people who have been infected naturally before have a more robust antibody response to the vaccine.
The study results are similar to the antibody levels seen in clinical trials of the mRNA vaccines, showing rapid production of antibodies. High levels of antibodies against the RBD seen in vaccinated individuals suggest good protection. The RBD is the portion of the spike protein that binds to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells.
Antibodies from natural infection do not have high levels against the RBD. This could be because the RBD epitope may be hidden to prevent host immune recognition. The less robust and variable antibody response to natural infection suggests immunity acquired by natural infection may not be as strong as that from vaccination. “We should not assume that previously infected individuals are immune or that they cannot transmit the virus,” write the authors.
Thus, vaccination induces a more robust antibody response, and even people who have been previously infected may benefit from the vaccine.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Philip L Felgner. et al. (2021) Substantial Differences in SARS-CoV-2 Antibody Responses Elicited by Natural Infection and mRNA Vaccination. bioRxiv, https://doi.org/10.1101/2021.04.15.440089, https://www.biorxiv.org/content/10.1101/2021.04.15.440089v2
- Peer reviewed and published scientific report.
Assis, Rafael, Aarti Jain, Rie Nakajima, Algis Jasinskas, Saahir Khan, Anton Palma, Daniel M. Parker, et al. 2021. “Distinct SARS-CoV-2 Antibody Reactivity Patterns Elicited by Natural Infection and MRNA Vaccination.” Npj Vaccines 6 (1). https://doi.org/10.1038/s41541-021-00396-3, https://www.nature.com/articles/s41541-021-00396-3