Researchers in the United States have shown that the antibody response induced by the Moderna mRNA-1273 vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a correlate of immune protection against coronavirus disease 2019 (COVID-19) in non-human primates.
A defined correlate can inform decisions to extend the use of approved vaccines, facilitate the development of new vaccine candidates, and help to determine potential mechanisms of protection.
Now, Seder and colleagues have shown that mRNA-1273 vaccination of non-human primates (NHPs) elicited robust circulating and mucosal antibody responses against the spike protein of SARS-CoV-2 in a dose-dependent manner.
The spike protein is the main structure the virus uses to infect host cells and the primary target of neutralizing antibodies following infection or vaccination.
This antibody response was associated with significantly reduced viral replication in lung fluid samples and nasal swabs taken from the animals after they were challenged with a highly pathogenic stock of SARS-CoV-2 (USA-WA1/2020).
The team also showed that the transfer of the vaccine-elicited antibodies to naïve hamsters was sufficient to protect the animals against disease when they were inoculated with SARS-CoV-2.
“Taken together, these data show that mRNA-1273 vaccine-induced humoral immune responses are a mechanistic correlate of protection against SARS-CoV-2 infection in NHP,” writes the team.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Vaccine development has been successful so far
Since the novel SARS-CoV-2 virus was first identified in Wuhan, China, in late December 2019, unprecedented efforts have been made to develop vaccines that will protect against COVID-19.
This resulted in the rapid approval of two mRNA-based vaccines: Moderna’s mRNA59 1273 and Pfizer/BioNTech’s BNT162b2.
Both vaccines, which encode the SARS-CoV-2 spike protein, were shown to be more than 94% effective at protecting against COVID-19 in Phase 3 trials. These vaccines are now being rolled out in many countries across the world.
Several other vaccines have demonstrated 60-80% efficacy against COVID-19, and a number of candidates are in the earlier stages of development.
The importance of immune correlates
Defining an immune correlate of protection is a critical aspect of COVID-19 vaccine development.
“This surrogate of vaccine efficacy can be used to inform potential dose reduction, advance approval of other vaccine candidates in lieu of Phase 3 efficacy data, extend indications for use to other age groups, and provide insights into the immune mechanisms of protection,” writes Seder and the team.
The NHP model of SARS-CoV-2 infection has been used to demonstrate the efficacy of several vaccine candidates. The assessment of immune correlates of viral load following primary infection has also been completed in NHP.
The model exhibits similar upper and lower airway infection and pathology to that observed in humans with mild COVID-19.
However, no studies to date have specifically defined immune correlates of protection in the upper and lower airways following immunization with any of the vaccines that have been approved for use in humans.
What did the researchers do?
Based on a study the researchers previously conducted into the immunogenicity and protection induced by mRNA-1273 vaccination in NHP, they hypothesized that serum antibody levels would serve as immune correlates of protection.
The team assessed how levels of humoral (antibody) immunity correlated with levels of viral replication in NHP challenged with highly pathogenic SARS-CoV-2 (USA-WA1/2020) after the animals had received the mRNA-1273 vaccine (0.3–100 µg).
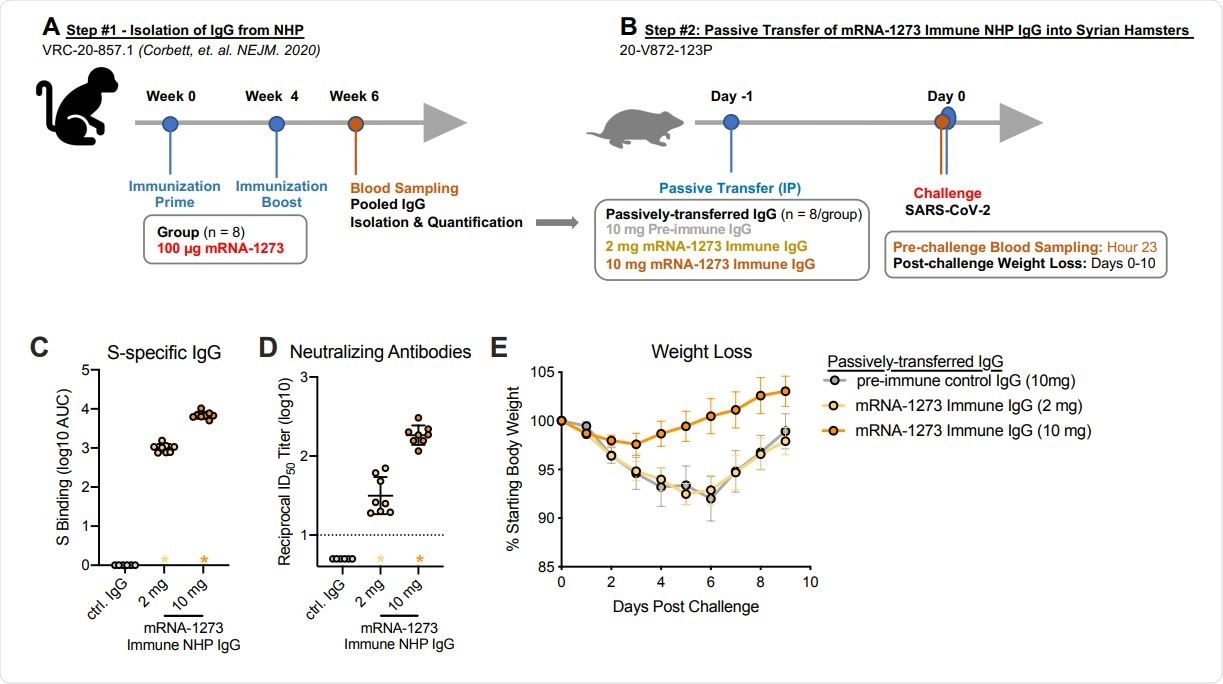
Passive transfer of mRNA-1273 immune NHP IgG into Syrian hamsters. (A) Sera were pooled from all NHP that received 100 µg of mRNA-1273 in a primary vaccination series. (B) mRNA-1273 immune NHP IgG (2 mg, yellow or 10 mg, orange) or pre-immune NHP IgG (10 mg, gray) was passively transferred to Syrian hamsters (n = 8/group) 24 hours prior to SARSCoV-2 challenge. Twenty-three hours post-immunization, hamsters were bled to quantify circulating S-specific IgG (C) and SARS-CoV-2 pseudovirus neutralizing antibodies (D). Following challenge, hamsters were monitored for weight loss (E). (C-D) Circles represent individual NHP. Bars and error bars represent GMT and geometric SD, respectively. Asterisks at the axis represent animals that did not receive adequate IgG via passive transfer and were thus excluded from weight loss analyses. (D) The dotted line indicates the neutralization assay limit of detection. (E) Circle and error bars represent mean and SEM, respectively.
What did they find?
Vaccination elicited robust circulating and mucosal antibody responses in a dose-dependent manner.
Following the SARS-CoV-2 challenge, a 10-fold increase in spike-specific antibodies was associated with an approximate 10-fold reduction in viral replication in bronchoalveolar lavage (BAL) samples and nasal swabs taken from the animals.
“Here, we establish that the level of spike-specific antibody elicited by mRNA-1273 vaccination correlates with control of upper and lower airway viral replication following SARS-CoV-2 challenge in NHP,” writes Seder and colleagues.
“These reductions may be sufficient to prevent moderate or severe lower airway infection,” they add.
Notably, even animals that were administered 1 and 3 µg vaccine doses exhibited significantly reduced viral replication in BAL, compared with control animals just 2 days post-challenge.
Furthermore, consistent with antibodies being a correlate of protection, the passive transfer of vaccine-induced antibodies from NHP to naïve hamsters mediated protection against disease following the SARS-CoV-2 challenge.
Spike-specific antibodies as a correlate of protection
“Together, these studies support spike-specific antibodies as a correlate of protection, highlight the ability of localized mucosal antibodies to control upper and lower airway viral replication, and confirm mRNA-1273-induced IgG to be sufficient for protection against SARS-CoV-2 infection in preclinical models,” write the researchers.
“These findings anticipate the correlates analysis comparing virus replication in nasal swabs with serum antibody that is being performed on samples from vaccinated subjects in Phase 3 clinical trials who experienced breakthrough infection,” says the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Seder R, et al. Immune Correlates of Protection by mRNA-1273 Immunization against SARS-CoV-2 Infection in Nonhuman Primates. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.04.20.440647, https://www.biorxiv.org/content/10.1101/2021.04.20.440647v1
- Peer reviewed and published scientific report.
Corbett, Kizzmekia S., Martha C. Nason, Britta Flach, Matthew Gagne, Sarah O’Connell, Timothy S. Johnston, Shruti N. Shah, et al. 2021. “Immune Correlates of Protection by MRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates.” Science 373 (6561). https://doi.org/10.1126/science.abj0299. https://www.science.org/doi/10.1126/science.abj0299.