Researchers in the UK have conducted a study showing the real-world effectiveness of Pfizer-BioNTech’s vaccine at protecting against symptomatic coronavirus disease 2019 (COVID-19) when it is administered in line with the original dosing schedule.
The team from NHS England & NHS Improvement in Leeds and the University of Manchester says the vaccine proved effective despite the wide circulation of the more transmissible B.1.1.7 variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Although Pfizer-BioNTech’s BNT162b2 mRNA vaccine has proved effective at preventing severe COVID-19 in clinical trials, less evidence has been provided regarding its effectiveness in real-world settings, particularly among older people.
Concerns regarding effectiveness have also arisen since the emergence of the highly transmissible and widely circulating SARS-CoV-2 variant B.1.1.7.
Now, in a study of more than 170,000 individuals (aged 80 to 83 years) and matched controls, Thomas Mason and colleagues have shown that neutralization of the variant was only slightly reduced following BNT162b2 vaccination.
The vaccine’s efficacy against SARS-CoV-2 infection was estimated to be around 55% after one dose and approximately 70% after a second dose administered 21 days later.
“The nationwide vaccination of older adults in England with the BNT162b2 mRNA vaccine reduced the burden of COVID-19,” writes the team.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Assessing efficacy in the real-world setting
Since the COVID-19 outbreak first began in Wuhan, China, in late December 2019, several vaccines protecting against SARS-CoV-2 infection are safe and effective in phase 3 clinical trials, with efficacy estimates for prevention of symptomatic COVID-19 ranging from 62% to 95%.
However, it is also important to assess vaccine efficacy following mass deployment in the real-world setting where trial exclusion criteria do not apply and where people may not adhere to dosing and handling protocols.
Early observational studies conducted in Israel, Scotland, and England have demonstrated that the real-world efficacy of the Pfizer-BioNTech BNT162b2 vaccine is consistent with that reported in clinical trials and is maintained against the more transmissible B.1.1.7. variant.
“However, such observational, non-randomized studies may be biased by systematic differences between intervention and control groups and between those receiving the intervention at different points in time,” writes Mason and colleagues.
Furthermore, the unprecedented speed of vaccination rollouts and the specific prioritization of vulnerable groups increases the risk of these biases, they add.
“The rapid rollout of the NHS vaccination program in England based on age thresholds offers an opportunity to make unbiased comparisons of outcomes between vaccinated and unvaccinated populations,” says the team.
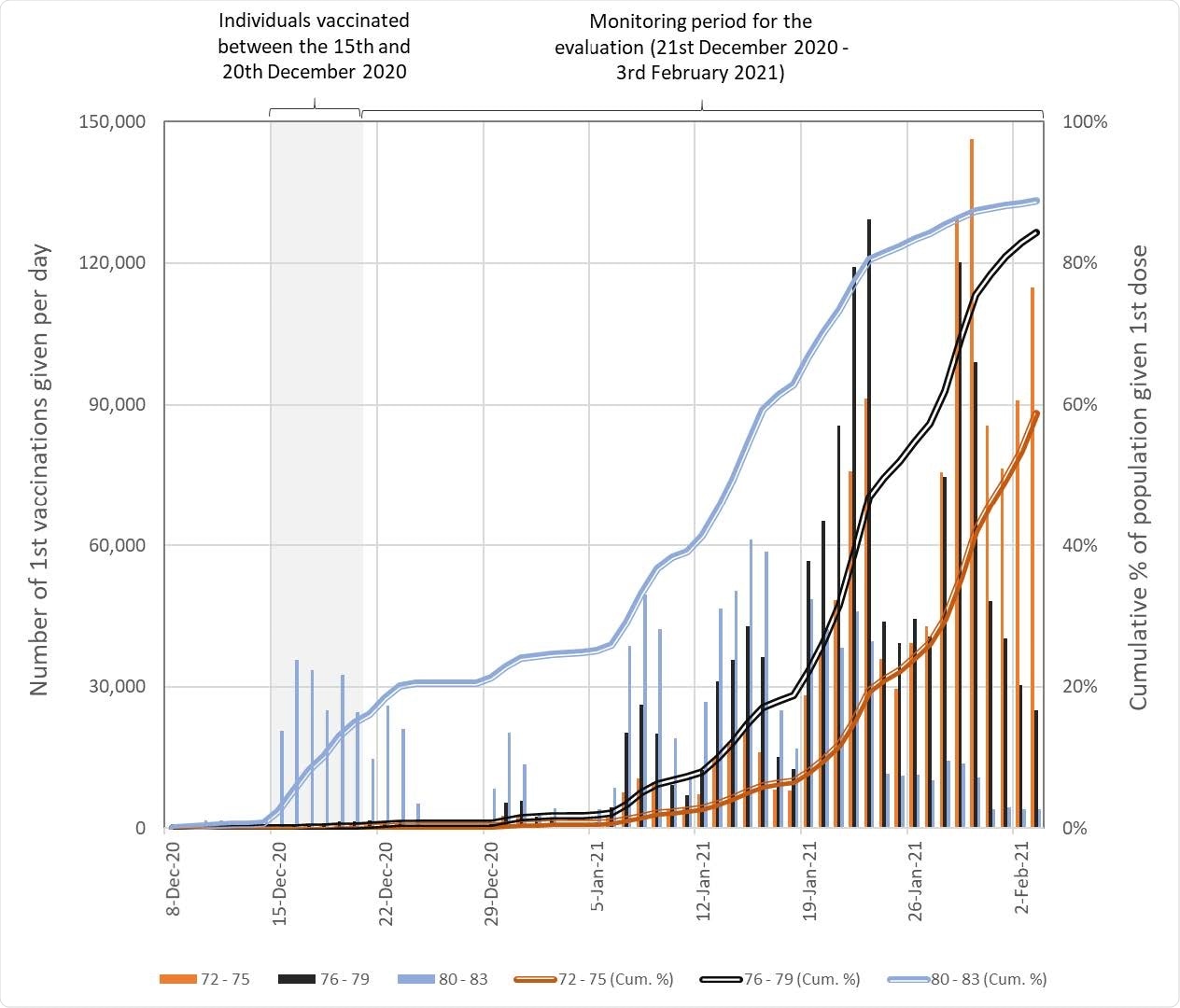
Numbers of people in England who received their first COVID-19 vaccination dose between the 8th December 2020 and the 3rd February 2021 by age group. The cumulative totals are relative to estimates of eligible population based on extracts from the National Health Application and Infrastructure Services (NHAIS) system as of the 15th November 2020. Prior to the 4th January 2021 all individuals received the BNT162b2 mRNA vaccine, after which individuals were vaccinated with either the BNT162b2 mRNA or ChAdOx1 adenovirus vector vaccines.
More about the study
The researchers exploited the age-based eligibility phasing in the early stages of the nationwide vaccination rollout in England to estimate the real-world effectiveness of BNT162b2 vaccination.
The team matched 170,226 vaccine recipients (aged 80 to 83 years) from the community setting outside of care homes with slightly younger (aged 76-79 years) individuals not yet eligible for vaccination on gender, area of residence, health status, living arrangements, acute illness, and history of seasonal flu vaccination.
“It is particularly important to assess real-world vaccine effectiveness in this older population group because severe COVID-19 is strongly age-associated and adaptive immune responses decline with age,” says Mason and the team.
The vaccine recipients received one dose between the 15th and 20th December 2020 and were scheduled to receive a second dose 21 days later.
The rates of COVID-19 infection and hospitalization were compared between the vaccinees and the matched controls over the 45 days following the date that of the first vaccine dose.
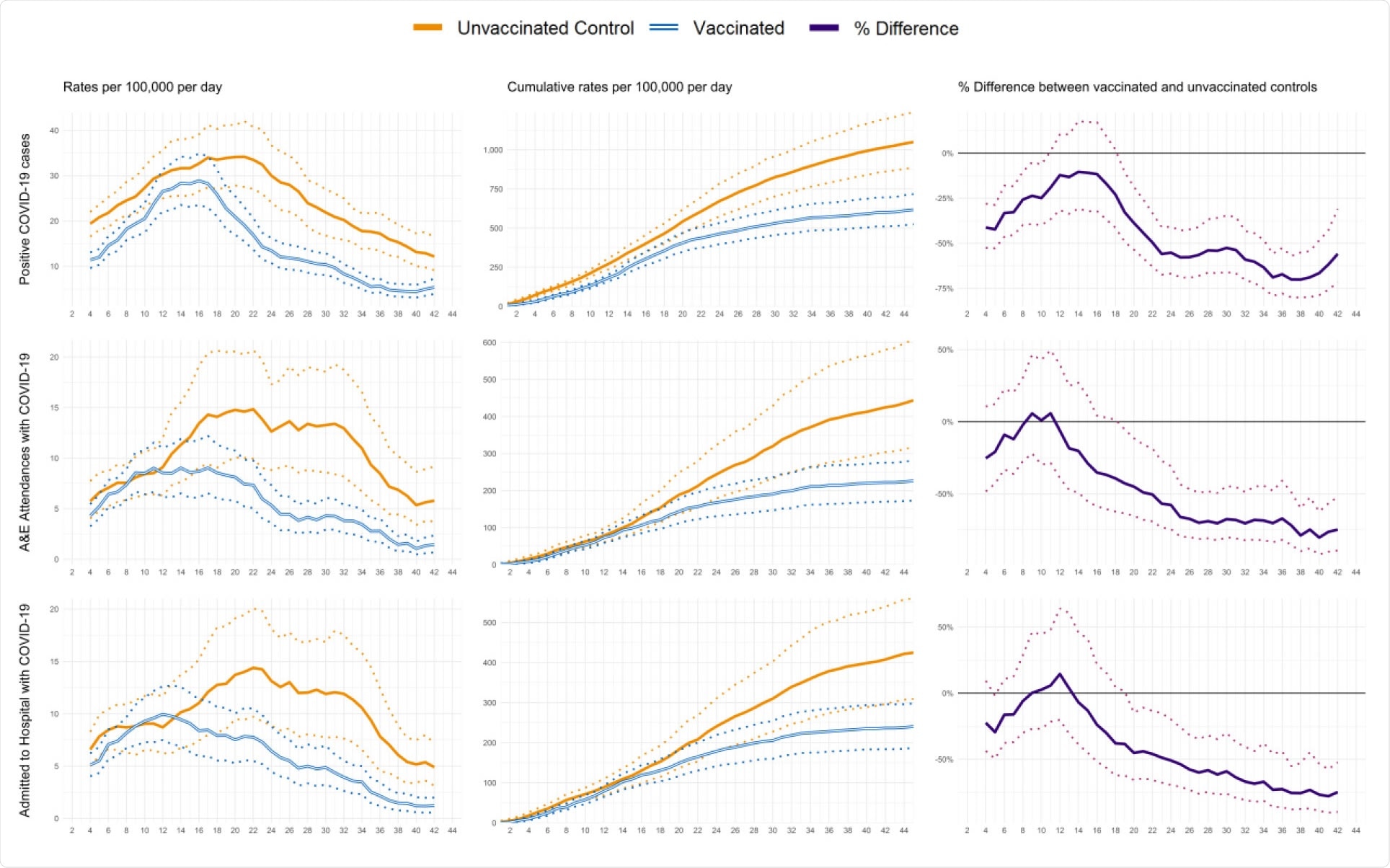
Profiles of positive COVID-19 infections and emergency hospital attendances and admissions by days since first dose of vaccination. The data represent people aged between 80 to 83 years who received their first dose of the BNT162b2 mRNA Covid-19 vaccine between the 15th and 20th December 2020 with comparison to their matched controls. 95% confidence intervals are displayed as dashed lines.
What were the efficacy results?
During the 45-day follow-up period, the average incidence of SARS-CoV-2 infection was 13.7 per day per 100,000 among the vaccinated individuals and 23.2 per day per 100,000 among the unvaccinated controls.
The rates of emergency hospital admissions and infection were 51.0% and 55.2% lower, respectively, for vaccinated individuals versus matched controls at 21 to 27 days following first vaccination.
The rates of emergency admissions and infections were 75.6% and 70.1% lower 35 to 41 days following first vaccination when 80% of participants had received their second dose.
“Collectively, these results are consistent with one dose of the BNT162b2 mRNA vaccine reducing events from 14 days after vaccination, with more effectiveness in reducing the severity of symptoms than preventing infection,” says Mason and colleagues.
“We provide evidence of high real-world effectiveness of the original dosing schedule of the BNT162b2 mRNA Covid-19 vaccine in preventing infections and hospitalizations, despite the widespread transmission of the B.1.1.7 variant shortly after the study population was vaccinated,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources