In the setting of potentially reduced vaccine efficacy against coronavirus disease (COVID-19) due to emerging viral variants, the deployment of effective treatments based on monoclonal antibodies may be an additional strategy to help control the pandemic. A new study by US researchers, currently available on the bioRxiv* preprint server, describes the action of a specific antibody known as DH1047.
The emergence of SARS-CoV (a causative agent of the original SARS outbreak) and SARS-CoV-2 (a causative agent of COVID-19) in the last two decades highlights an urgent need to expand our armamentarium against Sarbecoviruses, which is a group of RNA viruses (notorious for jumping from animals to humans) that includes two aforementioned pathogens.
Likewise, with the recent emergence of more transmissible, highly virulent, and neutralization resistant variants that can partly avoid existing countermeasures, there is a need to devise next-generation monoclonal antibodies that will be able to broadly neutralize current and future SARS-CoV-2 variants of concern.
These variants can diminish the efficacy of vaccines that are currently distributed; hence, real-time monitoring is needed to evaluate the performance of current vaccine protocols and therapies with monoclonal antibodies. These insights may prove indispensable for mitigating any future zoonotic event caused by the same group of viruses.
In agreement with the notion that the receptor-binding domain (RBD) of the spike glycoprotein contains a conserved epitope that is shared among SARS, SARS-like, SARS-CoV-2, and the variants of concern, researchers from the University of North Carolina at Chapel Hill and Duke University School of Medicine have identified a pan-coronavirus protective antibody – DH1047.
Evaluating protective potential in laboratory and animal studies
In order to appraise whether cross-reactive antibodies can neutralize divergent Sarbecoviruses, this research group has measured neutralizing activity against live viruses such as mouse-adapted SARS-CoV-2 2AA virus, SARS-CoV, bat coronavirus RsSHC014, and bat coronavirus WIV-1.
Furthermore, to evaluate the protective efficacy of the four RBD-specific cross-reactive IgG antibodies found in the previous step (designated as DH1235, DH1073, DH1046, and DH1047), they have used them to immunize aged mice passively and subsequently evaluated viral replication in lungs.
The binding epitope of DH1047 was then visualized and compared with the previously published structure of the complex with the SARS-CoV-2 spike ectodomain. At the same time, its protective efficacy was further appraised against SARS-CoV-2 B.1.351 variant and pre-emergent bat coronaviruses.
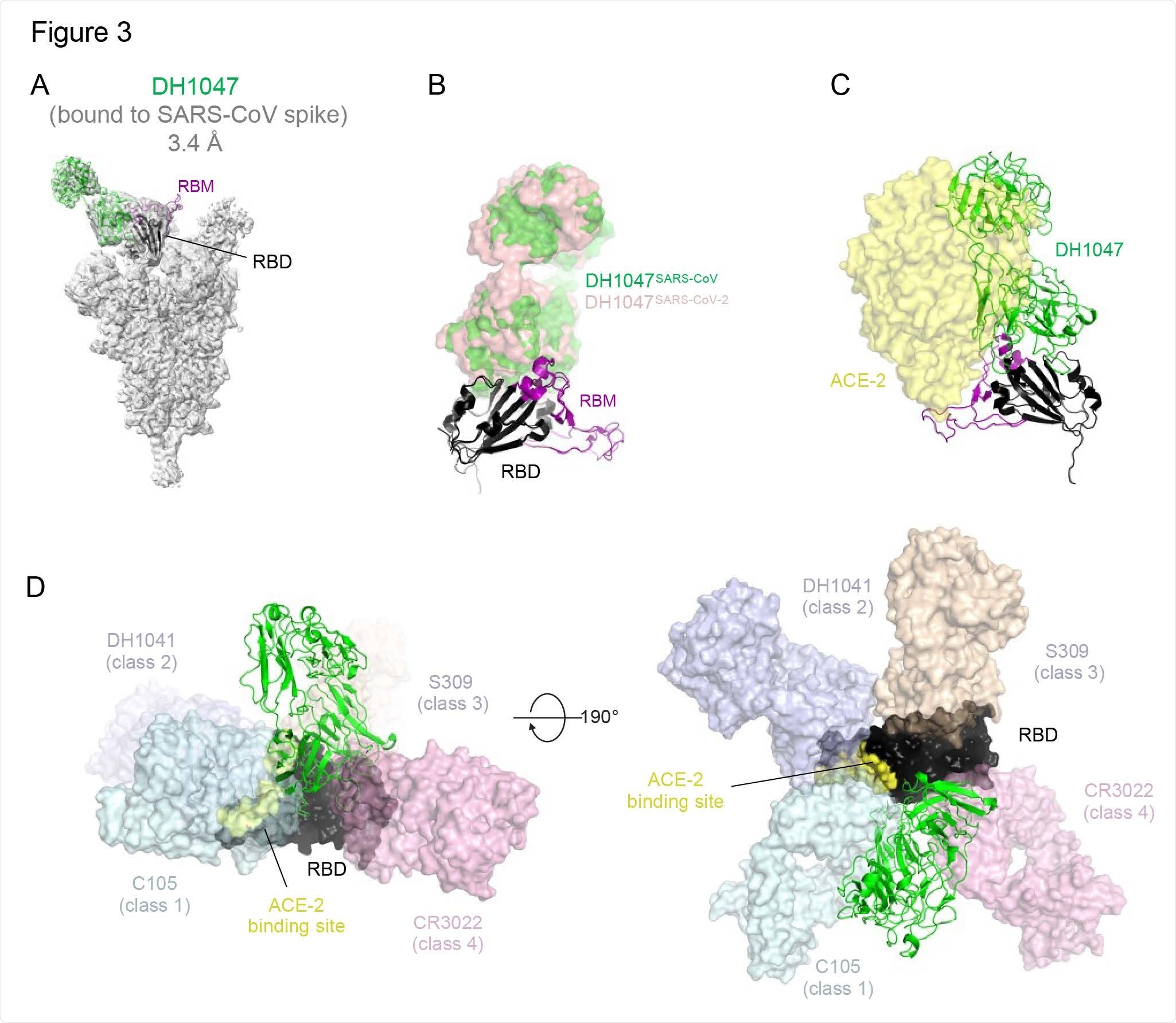
Cryo-EM structure of DH1047 bound to SARS-CoV spike. (A) Cryo-EM reconstruction of DH1047 Fab bound to SARS-CoV spike shown in grey, with the underlying fitted model shown in cartoon representation. DH1047 is colored green, the RBD it is bound to is colored black with the Receptor Binding Motif within the RBD colored purple. (B) Overlay of DH1047 bound to SARS-CoV-1 and SARS-CoV-2 (PDB ID: 7LDI) S proteins. Overlay was performed with the respective RBDs. DH1047 bound to SARS-CoV and SARS708 CoV-2 spike is shown in green and and salmon, respectively. (C) ACE2 (yellow surface representation, PDB 6VW1) binding to RBD is sterically hindered by DH1047. The views in panels B and C are related by a ~180º rotation about the vertical axis. (D) DH1047 binding relative to binding of other known antibody classes that bind the RBD. RBD is shown in black with the ACE2 footprint on the RBD colored yellow. DH1047 is shown in cartoon representation and colored green. The other antibodies and shown as transparent surfaces: C105 (pale cyan, Class 1, PDB ID: 6XCN and 6XCA), DH1041 (light blue, Class 2, PDB ID: 7LAA), S309 (wheat, Class 3, PDB ID:6WS6 and 6WPT) and CR3022 (pink, Class 4 , PDB ID: 6YLA)
Robust efficacy against zoonotic SARS-like viruses
In this study, the authors have demonstrated successful neutralization of SARS-CoV, bat coronaviruses RsSHC014 and WIV-1, as well as SARS-CoV-2 variants D614G, B.1.1.7 (UK variant), B.1.429 (California variant), B1.351 (South African variant) by a receptor-binding domain (RBD)-specific antibody DH1047.
Furthermore, prophylactic and therapeutic approaches with the use of DH1047 showed adequate protection against SARS-CoV, RsSHC014, WIV-1, and SARS-CoV-2 B1.351 variant infection in the mice model. Additional structural and attachment analysis demonstrated high-affinity binding of DH1047 to a specific epitope (or antigen molecule) highly conserved among Sarbecoviruses.
“We conclude that DH1047 is a broadly protective monoclonal antibody that has efficacy against pre-emergent, zoonotic SARS-like viruses from different clades, neutralizes highly transmissible SARS-CoV-2 variants, and protects against SARS-CoV-2 B.1.351”, say study authors in this bioRxiv paper.
Implications for treatment and future approaches
In the future, it will be pivotal to monitor both SARS and SARS2-like coronaviruses of zoonotic origin closely and actively observe if broad-spectrum antibodies like DH1047, ADG-2, and S2X259 maintain their inhibitory activity when confronted with pre-emergent viruses.
“We envision a system in which broad-spectrum antibodies like DH1047 could be tested for safety in small Phase I clinical trials so that in the event that a future SARS-like virus emerges, DH1047 could immediately be tested in larger efficacy trials at the site of an outbreak to potentially prevent the rapid spread of an emergent coronavirus”, further explain study authors.
In addition, taking into account that DH1047 displayed vigorous in vivo protection against the SARS-CoV-2 B.1.351 variant of concern, this monoclonal antibody could be used as a potential therapeutic in our combat against the ongoing COVID-19 pandemic. These findings support its early administration, as this may well be a critical step in preventing severe disease outcomes.