The ongoing global pandemic of coronavirus disease 2019 (COVID-19) has instigated a rapid response in terms of vaccine and drug development targeting the causative agent, SARS-CoV-2. One of its salient targets is the spike glycoprotein, which recognizes the receptor and mediates viral entry into the cell.
A myriad of biophysical and structural studies (alongside molecular dynamics simulations) have demonstrated that the SARS-CoV-2 spike glycoprotein is highly dynamic. More importantly, recent studies that used cryo-electron microscopy for structural analysis have demonstrated cryptic pockets in the receptor-binding domain (RBD) and N-terminal domain of the virus, which hold promise as potential target epitopes.
In recent years, simulations on different pathogens have utilized proteins with small organic probes in order to incite the energetically unpropitious opening of hydrophobic cryptic pockets and, in turn, reveal novel druggable sites.
In this new study, a research group led by Dr. Lorena Zuzic from the Bioinformatics Institute (A*STAR) in Singapore and the University of Manchester in the UK built a glycosylated model of the SARS-CoV-2 spike glycoprotein. They simulated it in the presence of benzene probes – with the end goal to enhance the sampling of new cryptic pockets as potential targets of peptides, small molecules or monoclonal antibodies.
Unveiling the effect of glycosylation
This research group first developed a complete model of the SARS-CoV-2 spike glycoprotein in the open “up-down-down” RBD configuration and then investigated how glycosylation influences spike glycoprotein dynamics by generating three different glycoforms.
More specifically, they were made with the most dominant glycan species in accordance with the mass spectrometry data, but also with only high-mannose glycans to cover the unprocessed glycoform, as well as without any glycans. In addition, to simulate the spike glycoprotein in its native membrane environment, the researchers have embedded the models in an endoplasmic reticulum-Golgi intermediate compartment (ERGIC) membrane.
Then, they have performed a series of 200 nanosecond simulations of such membrane-embedded, full-length spike glycoprotein models with a 0.2 molar concentration of benzene molecules within the bulk solvent. Structural alignment was pursued in order to provide a detailed outline of the mapped pocket.
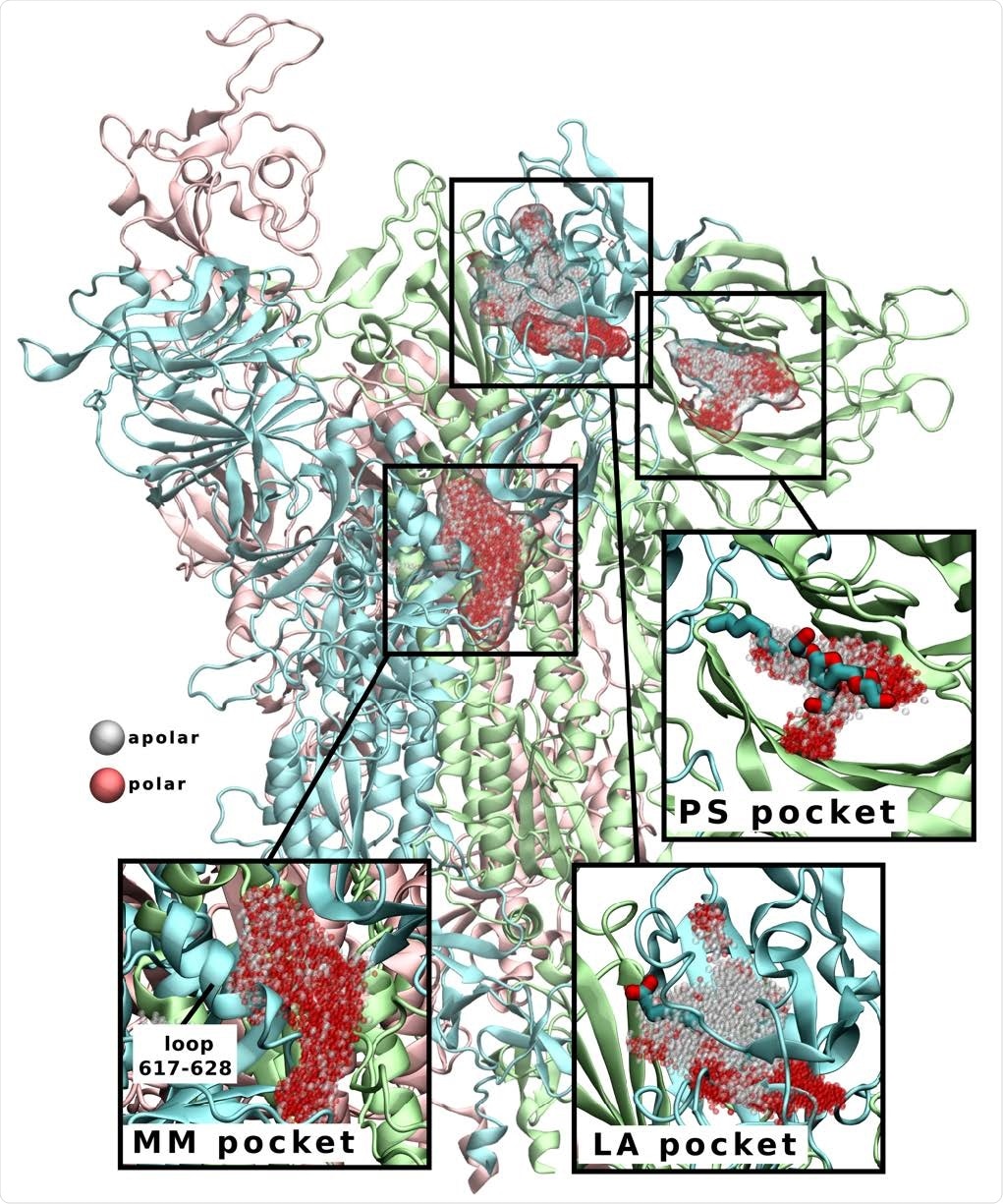
S protein cryptic pockets revealed during simulations in the presence of benzene. Linoleic acid (LA) and polysorbate (PS) pockets are named according to their bound ligands (shown in licorice representation in insets), as their binding sites have previously been identified by cryo-EM. Multimerization (MM) pocket is a surface groove that extends down the interchain interface and underneath the 617-628 loop (labeled in inset). Pockets are shown as clusters of grey (apolar) and red (polar) spheres.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Specificities of a novel SARS-CoV-2 cryptic pocket
A novel cryptic pocket with a rather promising druggable trait has been located in this paper, which is involved in the formation of spike glycoprotein multimers on the surface of the virus. Furthermore, this pocket was identified in systems emulating immature and mature glycosylation states, implying its druggability may not depend on the SARS-CoV-2 maturation stage.
An interesting finding is that the aforementioned pocket also harbors D614G mutation pivotal for SARS-CoV-2 fitness and within close proximity to mutations found in the novel SARS-CoV-2 variants B.1.1.7 and B.1.1.28 – both linked not only to increased transmissibility but also more severe COVID-19.
This specific methodological approach also managed to successfully recapitulate hydrophobic pockets in the RBD and N-terminal domain associated with lipid or detergent binding in antecedent cryo-electron microscopy studies, lending further credence to these findings.
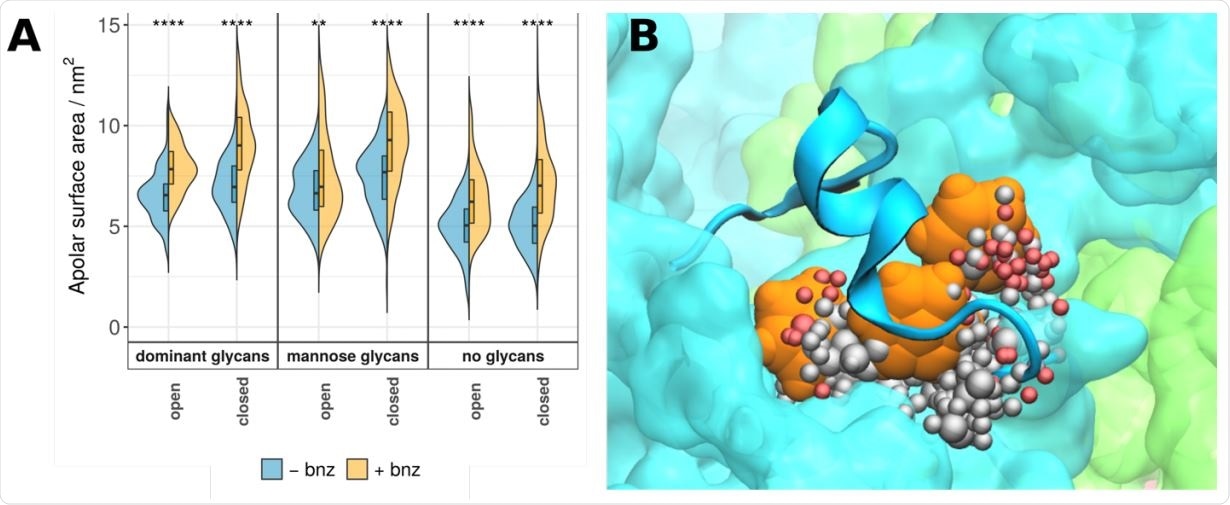
Chemical properties of the MM pocket. (A) Apolar surface areas of the MM pocket are shown as violin plots and labelled as in Figure 2. Similar to positive controls, the addition of benzene to the system increases overall apolar component of the pocket in question. System names are as described in Figure 2. (B) A portion of the MM pocket packed underneath the 617-628 loop. Three benzene molecules (in orange) are able to interact with the protein surface and occupy a predominantly hydrophobic binding pocket (spheres representing the pocket are colour coded as in Figure 1).
Towards a rational design of small-molecule drugs
Overall, this robust research endeavor underscores the utility and feasibility of the benzene mapping approach in detecting potential druggable sites situated on the surface of SARS-CoV-2 targets.
“In addition to successfully reproducing two independently identified cryptic pockets, we also uncovered a novel and potentially druggable pocket on the spike glycoprotein ectodomain surface,” say study authors in this bioRxiv paper.
“Simulations of different spike glycoforms indicated that cryptic pockets are present in all glycosylation states, suggesting that these pockets could be targeted in all stages of viral maturation,” they add.
In conclusion, together with ongoing efforts in vaccine development and antibody-based treatment, a rational design of small-molecule drugs that target spike glycoprotein pockets may be another essential tool in our fight against the COVID-19 pandemic.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zuzic, L. et al. (2021). Uncovering cryptic pockets in the SARS-CoV-2 spike glycoprotein. bioRxiv. https://doi.org/10.1101/2021.05.05.442536, https://www.biorxiv.org/content/10.1101/2021.05.05.442536v1
- Peer reviewed and published scientific report.
Zuzic, Lorena, Firdaus Samsudin, Aishwary T. Shivgan, Palur V. Raghuvamsi, Jan K. Marzinek, Alister Boags, Conrado Pedebos, et al. 2022. “Uncovering Cryptic Pockets in the SARS-CoV-2 Spike Glycoprotein.” Structure 30 (8): 1062-1074.e4. https://doi.org/10.1016/j.str.2022.05.006. https://www.cell.com/structure/fulltext/S0969-2126(22)00180-0.