COVID-19 (coronavirus disease 2019) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In our ongoing effort to combat COVID-19, it is vital to increase the testing of SARS-CoV-2 and highly transmissible variants.
The emergence of SARS-CoV-2 variants brings with it new challenges and concerns pertaining to vaccination efficacy, diagnostic sensitivity, and public health responses. In light of these variants and the prediction that COVID-19 will remain in circulation alongside other seasonal or endemic respiratory viruses that present similar symptoms, such as influenza, multiplexed diagnostic testing is required.
Recent research published on the medRxiv* server has developed a portable, magnetofluidic cartridge platform, for automated PCR testing in <30 min, for rapid multiplexed screening of pathogens. A multidisciplinary team of researchers at Johns Hopkins University and the Johns Hopkins University School of Medicine developed these cartridges for multiplexed detection of SARS-CoV-2 with distinctive variant mutations or with Influenza A and B.
Although virus variants and infectious pathogens can be identified using genomic sequencing, the technology is too expensive and complex to be widely adopted due to the necessary equipment and data processing required to conduct the sequencing procedures. The nucleic acid amplification tests (NAATs) can be utilized as an alternative, but they present significant issues, including a long turnaround time and high cost per test.
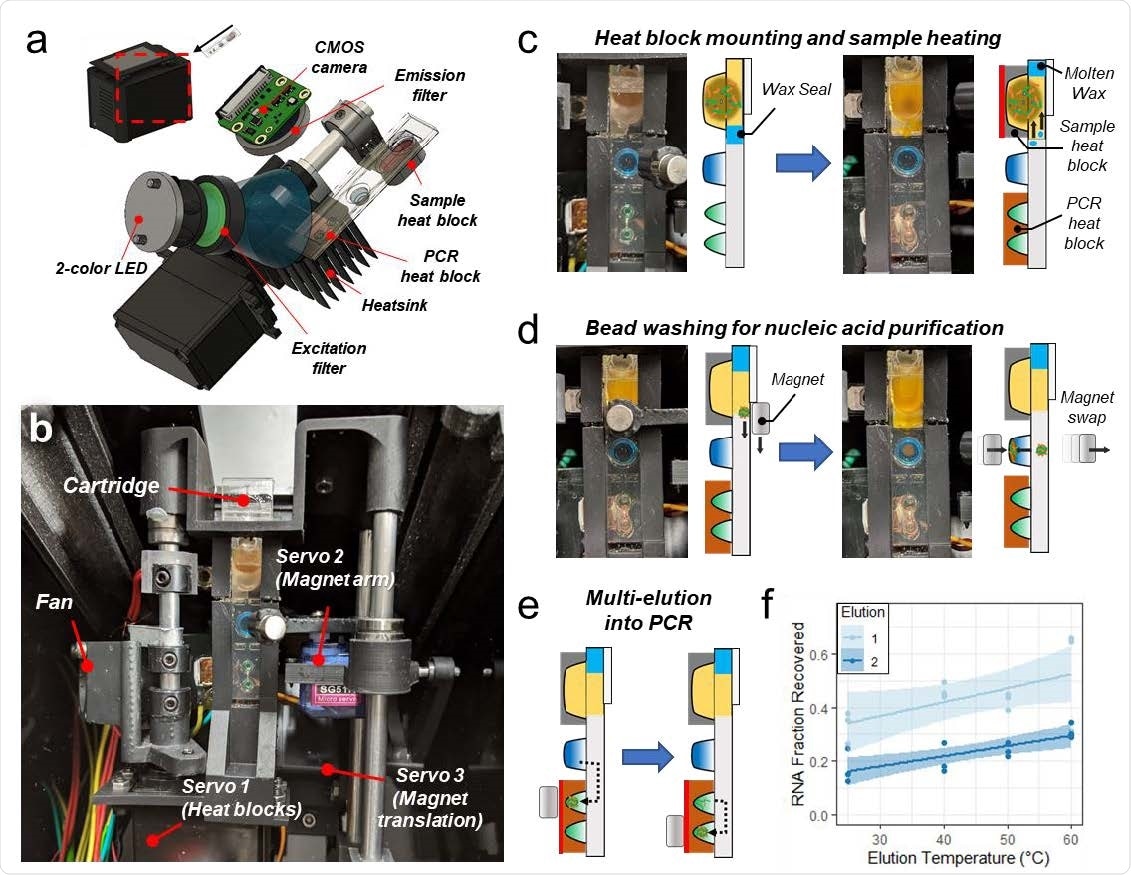
To address the need for affordable and accessible multiplexed NAATs with a fast turnaround time, the researchers in this study developed ‘an imaging-based portable droplet magnetofluidic cartridge platform,’ where the magnetic beads move through discrete droplets of reagents to capture, purify, and transfer analytes for amplification and/or detection.
“Magnetic transfer of beads along a hydrophobic surface within the cartridges obviates the need for precision fluidic channels and flow controllers that increase the complexity and cost of other automated NAAT platforms.”
Previous studies have used the magnetofluidic cartridges limited to a single reaction well per cartridge. In this work, the researchers achieved higher levels of multiplexed detection through a combination of duplexed PCR probe assays and a novel multi-elution aliquoting scheme to distribute the nucleic acids.
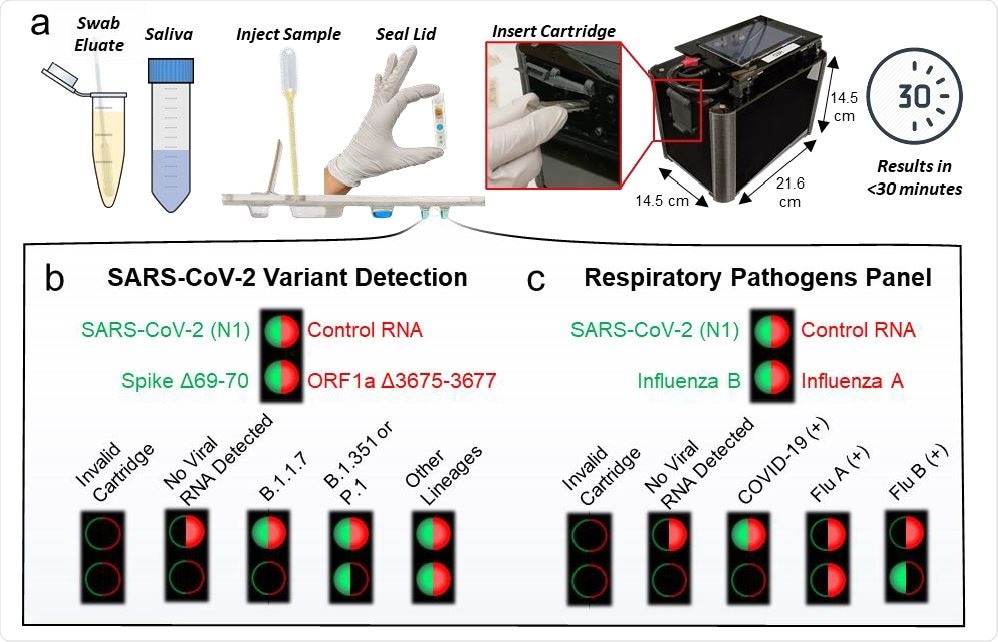
Cartridge platform operation. a, Nasal swab eluate or saliva is injected directly into the cartridge with magnetic beads followed by sealing the cartridge and inserting it into the instrument. After magnetofluidic sample preparation and PCR, the instrument reports the assay results on the built-in touchscreen within 30 minutes. b, Each PCR well contains two fluorescent probes in the FAM (green/left) or Cy5 (red/right) spectrum. Cartridges include a duplexed assay for the conserved N1 SARS-CoV-2 sequence and control RNA in the first well. The cartridge designed for detection of SARS-CoV-2 variants includes a duplexed PCR assay in the second well with probes spanning regions that contain deletions found in variants of concern. A lack of amplification in the second well indicates the presence of a mutation and can be used to classify the type of variant present. c, Cartridges designed for multiplexed detection of respiratory pathogens have a duplexed Influenza A and Influenza B PCR assay in the second well.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The highlight here is that this platform enables sample-to-answer RT-PCR in under 30 minutes with detection of up to 4 genetic targets per test. The researchers designed two different assay cartridges: 1) for SARS-CoV-2 detection and differentiation of its variants, and 2) for multiplexed screening of SARS-CoV-2 with Influenza A, and Influenza B. These assays test with extracted RNA from clinical samples, nasopharyngeal swab eluates, and saliva samples.
The researchers demonstrated the platform with a limit of detection down to 2 copies/µL SARS-CoV-2 RNA with successful identification of B.1.1.7 and B.1.351 variants.
Cartridge platform operation. a, Nasal swab eluate or saliva is injected directly into the cartridge with magnetic beads followed by sealing the cartridge and inserting it into the instrument. After magnetofluidic sample preparation and PCR, the instrument reports the assay results on the built-in touchscreen within 30 minutes. b, Each PCR well contains two fluorescent probes in the FAM (green/left) or Cy5 (red/right) spectrum. Cartridges include a duplexed assay for the conserved N1 SARS-CoV-2 sequence and control RNA in the first well. The cartridge designed for detection of SARS-CoV-2 variants includes a duplexed PCR assay in the second well with probes spanning regions that contain deletions found in variants of concern. A lack of amplification in the second well indicates the presence of a mutation and can be used to classify the type of variant present. c, Cartridges designed for multiplexed detection of respiratory pathogens have a duplexed Influenza A and Influenza B PCR assay in the second well.
They validated the multiplexed SARS-CoV-2/Flu assay using archived clinical nasopharyngeal swab eluates. The platform performed with good sensitivity/specificity (98.1%/95.2%, 85.7%/100%, 100%/98.2%, respectively), for SARS-CoV-2, Influenza A, and Influenza B. Notably, when tested with saliva, it demonstrated successful detection of all SARS-CoV-2 positive samples with no false positives.
Using simple thermoplastic cartridges, this study demonstrates great potential as a highly cost-effective and scalable solution to rapid testing. Furthermore, the platform is compact and user-friendly and compatible with a wide variety of sample types, including PBS, universal transport media, and saliva. One of the few limitations to the current platform is that the cartridges are not shelf-stable at room temperature.
“Our platform enables detection of multiple SARS-CoV-2 variants or other respiratory pathogens, while traditional rapid lateral-flow antigen tests and isothermal tests typically lack the ability to multiplex detection of several pathogens without either setting up multiple separate tests or requiring further post-processing steps for detection.”
The platform presented in this study is an essential opportunity to expand access to screening SARS-CoV-2 variants. With this multiplexed respiratory pathogen testing can be performed in any setting with minimal training and immediate on-site reporting of results, which help in correct surveillance recording and immediate notification of the result to the patient to initiate quarantine and healthcare.
These findings provide critical information for informed policy decisions that will assist in mitigating viral spread. Additionally, the post-pandemic multiplexed screening of respiratory infections will be essential in appropriately managing outbreak patients that present with similar respiratory symptoms, it is noted in the study.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Magnetofluidic platform for rapid multiplexed screening of SARS-CoV-2 variants and respiratory pathogens, Alexander Y. Trick, Fan-En Chen, Liben Chen, Pei-Wei Lee, Alexander C. Hasnain, Heba H. Mostafa, Karen C. Carroll, Tza-Huei Wang, medRxiv 2021.05.10.21256995; doi: https://doi.org/10.1101/2021.05.10.21256995, https://www.medrxiv.org/content/10.1101/2021.05.10.21256995v1
- Peer reviewed and published scientific report.
Trick, Alexander Y., Fan‐En Chen, Liben Chen, Pei‐Wei Lee, Alexander C. Hasnain, Heba H. Mostafa, Karen C. Carroll, and Tza‐Huei Wang. 2022. “Point‐Of‐Care Platform for Rapid Multiplexed Detection of SARS‐CoV‐2 Variants and Respiratory Pathogens.” Advanced Materials Technologies 7 (6): 2101013. https://doi.org/10.1002/admt.202101013. https://onlinelibrary.wiley.com/doi/10.1002/admt.202101013.