Researchers in Germany have demonstrated the preclinical effectiveness of a next-generation coronavirus disease 2019 (COVID-19) vaccine at enhancing antibody responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The researchers from CureVac AG in Tübingen and the Friedrich-Loeffler-Institute in Greifswald-Insel Riems say that compared with their previous vaccine candidate – CvnCoV – the second-generation CV2CoV vaccine accelerated induction of neutralizing antibody titers in rats just two weeks following the first dose.
The vaccine also elicited significant cross-neutralization of circulating SARS-CoV-2 variants of concern.
“This vaccine was developed to further increase vaccine efficacy, thereby setting the stage to tackle future challenges of the SARS-CoV-2 pandemic,” says Susanne Rauch and colleagues.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The challenges faced in COVID-19 vaccine development
Since the COVID-19 outbreak first began in late December 2019, unprecedented efforts to develop vaccines against SARS-CoV-2 have led to the authorization of thirteen vaccines, with another 93 currently in the pipeline.
However, many challenges remain. Insufficient vaccine supplies have hampered mass immunization programs across the globe. New variants of SARS-CoV-2 have also emerged that are less susceptible to the neutralizing antibodies elicited by vaccines based on the original viral strain.
Rauch and colleagues say the emergence of variants of concern with the potential to evade pre-existing immunity highlights the need for continuous improvement and adaptation of vaccines.
“mRNA vaccines represent a promising technology to meet this need: they are based on a highly versatile, adaptable platform technology whose efficacy as a vaccine against SARS-CoV-2 has been demonstrated resulting in authorized vaccines,” they write.
More about the CvnCoV and CV2Cov vaccines
The researchers say that CureVac’s mRNA technology showed promise in both preclinical and clinical studies and provided the basis for CvnCoV. This lipid nanoparticle-formulated mRNA vaccine encodes for the full-length viral spike protein.
The spike protein is the main structure SARS-CoV-2 uses to infect host cells and the primary target of neutralizing antibodies following vaccination.
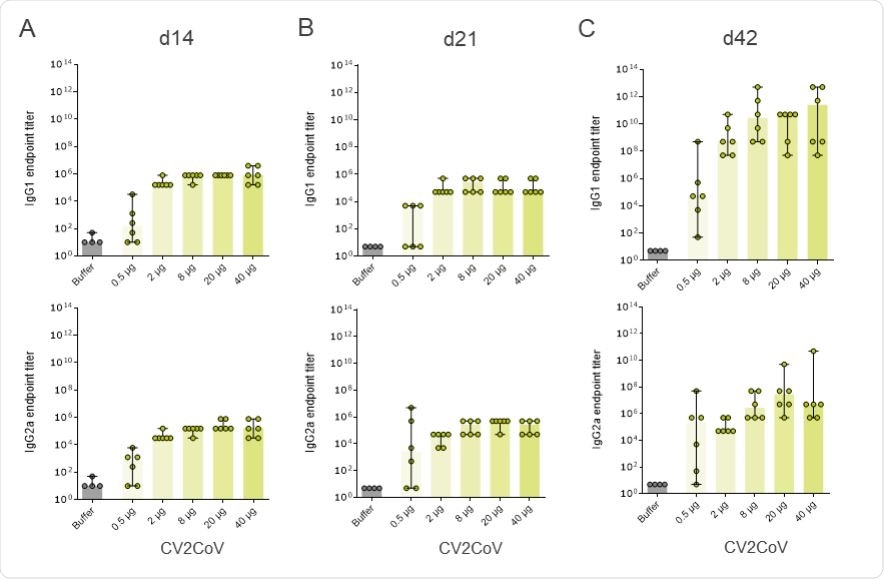
CV2CoV triggers high levels of binding antibody responses in rats. Female and male Wistar rats (n=6/group) were vaccinated IM on day 0 and d21 with five different doses ranging from 0.5 µg – 40 µg of CV2CoV. Wistar rats (n=4) vaccinated with 0.9% NaCl (Buffer) on day 0 and day 21 served as negative control. SRBD protein specific binding antibodies, displayed as endpoint titres for IgG1 and IgG2a in serum upon one vaccination ((A) day 14 and (B) day 21) or two vaccinations ((C) day 42). Each dot represents an individual animal, bars depict the median.
In Phase 1 clinical trials, CVnCoV vaccination resulted in full seroconversion two weeks following a second dose and elicited neutralizing antibody titers comparable to those of convalescent sera.
The CVnCoV vaccine is currently being tested in phase 2b/3 studies of efficacy, safety, and immunogenicity.
“Beyond this, dose sparing supported by a more immunogenic vaccine might facilitate providing more doses for a worldwide need,” say the researchers.
The team says the second-generation CV2CoV vaccine contains non-structural elements that were introduced to increase and prolong spike protein expression and thereby improve immunogenicity.
“Despite promising results of CVnCoV in both preclinical models and early clinical evaluations, we have continued the development of our mRNA technology with the aim to create a vaccine against SARS-CoV-2 with enhanced immunogenicity,” writes Rauch and colleagues.
What has the current study found?
Now, the team has shown that the CV2CoV vaccine indeed supports higher levels of spike protein expression in cell culture, compared with the original CVnCoV candidate.
Flow cytometry-based analysis showed that HeLa cells transfected with CV2CoV mRNA exhibited a 3.3-fold increase and 1.8-fold increase in the intracellular expression and cell surface expression of the spike, respectively, compared to transfection with CVnCoV mRNA.
The team also found that Wistar rats vaccinated with 0.5µg, 2µg or 8µg of CV2CoV elicited a strong dose-dependent antibody response against the spike protein’s receptor-binding domain (RBD).
These RBD-binding antibodies developed rapidly and were detectable two weeks following a single injection across all dosage groups and a clear boost effect was detectable following a second vaccination.
Importantly, CV2CoV also induced significant, dose-dependent neutralizing antibody titers just two weeks following a single vaccination at a dose of 2µg or higher.
By contrast, preclinical studies of rats, hamsters and non-human primates have shown that two vaccinations of CVnCoV are required to induce significant neutralizing titers, thereby providing evidence for the enhanced characteristics of CV2CoV, says the team.
Furthermore, CV2CoV’s ability to induce robust neutralizing activity was highlighted by its cross-neutralization of three different SARS-CoV-2 variants, including the B.1.1.298, B.1.1.7 and B.1.351 that emerged in Denmark, the UK and South Africa, respectively.
What do the authors conclude?
Rauch and colleagues say the CV2CoV represents a highly promising second-generation vaccine with improved characteristics, including accelerated induction of neutralizing antibody titers two weeks after the first vaccination.
“Its ability to induce high levels of antibodies against SARS-CoV-2 at low doses supports a scenario in which vaccination with CV2CoV allows dose sparing that might be able to contribute to reducing worldwide vaccine shortage,” they write.
“Overall, CV2CoV represents a highly promising candidate for further clinical development to help meet some of the challenges of the SARS-CoV-2 pandemic,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Rauch S, et al. CV2CoV, an enhanced mRNA-based SARS-CoV-2 vaccine candidate, supports higher protein expression and improved immunogenicity in rats. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.05.13.443734, https://www.biorxiv.org/content/10.1101/2021.05.13.443734v1
- Peer reviewed and published scientific report.
Roth, Nicole, Jacob Schön, Donata Hoffmann, Moritz Thran, Andreas Thess, Stefan O. Mueller, Benjamin Petsch, and Susanne Rauch. 2022. “Optimised Non-Coding Regions of MRNA SARS-CoV-2 Vaccine CV2CoV Improves Homologous and Heterologous Neutralising Antibody Responses.” Vaccines 10 (8): 1251.https://doi.org/10.3390/vaccines10081251. https://www.mdpi.com/2076-393X/10/8/1251.