Despite the unprecedented impact of COVID-19 throughout the world, the approval of mRNA vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has offered new hope in countries worst affected. Studies have shown over 90% immunogenicity and efficacy rates for mRNA vaccines in the immunocompetent population globally. However, the response of patients who have immune-mediated inflammatory diseases (IMIDs) and are on immunomodulatory medications is not clear. Patients with IMID are said to have attenuated immune responses to seasonal flu vaccination.
Researchers from Germany and the USA recently investigated the cellular and humoral immune responses of patients with IMIDs on immunomodulatory treatment to mRNA COVID-19 vaccines. The research is published on the medRxiv* preprint server.
As part of the study, 51 patients with IMID at NYU Langone Health receiving the BNT162b2 mRNA vaccine were evaluated at baseline and after the second dose of the vaccine. About 26 healthy individuals acted as controls. The researchers analyzed Immunoglobulin G (IgG) antibody responses to the SARS-CoV-2 spike protein. Cellular immune response to the virus was further analyzed using high-parameter spectral flow cytometry. A second independent cohort of 31 patients with IMID and 182 controls from Erlangen, Germany, were also assessed for humoral immune responses.
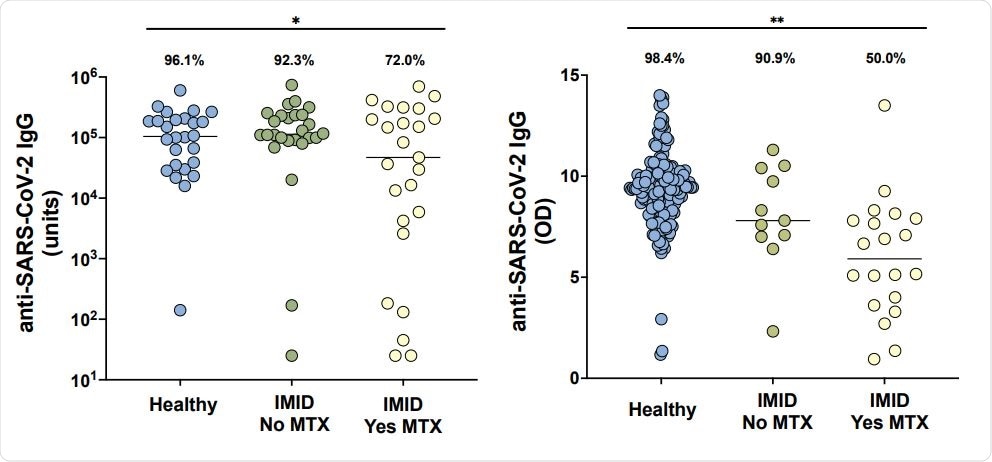
Anti-SARS-CoV-2 IgG levels in cohorts from New York City (A) and Erlangen (B) in healthy participants without immune-mediated inflammatory diseases (IMID; blue), IMID patients not receiving methotrexate (MTX; green), and IMID patients treated with MTX (yellow). Solid lines represent mean titer of each group. For the New York City cohort (A), adequate response is defined as greater than 5000 units and for the Erlangen cohort (B), adequate response is defined as greater than 5.7 (OD450nm), two standard deviations of the mean of controls. Percentages and group comparisons using chi squared test of independence reflect proportion of those achieving an adequate response within each group. * indicates p value less than .05. **indicated p value less than .001.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Results show reduced immunogenicity to BNT162b2 vaccination in IMID patients on methotrexate
The results showed that individuals with IMID on methotrexate showed up to a 62% reduced rate of immunogenicity to the BNT162b2 vaccination. Those who were on anti-cytokine or non-methotrexate oral medications showed similar immunogenicity levels compared to healthy controls who had over an immunogenicity rate of over 90%.
Similarly, the BNT162b2 vaccination did not induce an activated CD8+ T cell response in patients with IMID on methotrexate, unlike patients with IMID who were not receiving methotrexate and healthy controls.
“In two geographically independent cohorts of IMID patients, we found that methotrexate, a widely used immunomodulator for the treatment of several IMIDs, adversely affected humoral and cellular immunogenicity to COVID-19 mRNA vaccines.”
Patients with IMID on methotrexate may need different strategies to improve immunization efficacy
This study looked at the humoral and cellular immune responses to 2 doses of the BNT162b2 mRNA vaccine for COVID-19 in patients with IMID on immunomodulators and compared them with healthy controls. The findings showed that in 2 geographically independent cohorts of IMID patients, methotrexate - a widely used immunomodulator in the treatment of IMIDs - adversely impacted humoral as well as cellular immune responses of the patients to COVID-19 mRNA vaccines.
Although the exact level of immunogenicity that correlates with the efficacy of vaccines is yet to be established, these findings indicate that different strategies may need to be implemented in patients with IMID on methotrexate to improve their immunization efficacy against the SARS-CoV-2 virus.
The authors also recognize that the definition of adequate humoral and cellular immune response may need to be modified in the future when correlation with efficacy becomes evident. However, even after adjusting for these shortcomings, the negative effects of methotrexate on immunogenicity were still clear.
Strategies such as dose modification of methotrexate, temporary discontinuation of methotrexate, or additional vaccine doses need to be tested in patients with IMID for boosting immunogenicity of COVID-19 vaccines. Further studies are needed to explore the effectiveness of these alternative approaches on the immunogenicity of mRNA vaccine.
“Taken together, our results suggest that the optimal protection of patients with IMID against COVID-19 will require further studies to determine whether additional doses of vaccine, dose modification of methotrexate, or even temporary discontinuation of this drug can boost immune response as has been demonstrated for other viral vaccines in this patient population.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Methotrexate Hampers Immunogenicity to BNT162b2 mRNA COVID-19 Vaccine in Immune-Mediated Inflammatory Disease, Rebecca H. Haberman, Ramin Sedaghat Herati, David Simon, Marie Samanovic, Rebecca B. Blank, Michael Tuen, Sergei B. Koralov, Raja Atreya, Koray Tascilar, Joseph R. Allen, Rochelle Castillo, Amber R. Cornelius, Paula Rackoff, Gary Solomon, Samrachana Adhikari, Natalie Azar, Pamela Rosenthal, Peter Izmirly, Jonathan Samuels, Brian Golden, Soumya Reddy, Markus Neurath, Steven B. Abramson, Georg Schett, Mark J. Mulligan, Jose U. Scher, medRxiv, 2021.05.11.21256917; doi: https://doi.org/10.1101/2021.05.11.21256917, https://www.medrxiv.org/content/10.1101/2021.05.11.21256917v1
- Peer reviewed and published scientific report.
Haberman, Rebecca H, Ramin Herati, David Simon, Marie Samanovic, Rebecca B Blank, Michael Tuen, Sergei B Koralov, et al. 2021. “Methotrexate Hampers Immunogenicity to BNT162b2 MRNA COVID-19 Vaccine in Immune-Mediated Inflammatory Disease.” Annals of the Rheumatic Diseases, May, annrheumdis-2021-220597. https://doi.org/10.1136/annrheumdis-2021-220597. https://ard.bmj.com/content/80/10/1339.