A team of scientists from the United States has recently developed a formulation of toll-like receptor 9 (TLR9) agonist and aluminum hydroxide to increase the immunogenicity of spike receptor-binding domain (RBD) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The study findings reveal that RBD-based vaccines adjuvanted with the formulation can induce strong anti-SARS-CoV-2 neutralizing immunity in young and aged mice. The study is currently available on the bioRxiv* preprint server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
As of May 24, 2021, globally, there have been over 167 million confirmed infections of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including over 3.46 million deaths, registered to the World Health Organization. Achieving herd immunity through rapid vaccination is the key to control SARS-CoV-2 spread and save the world population from significant morbidity and mortality.
Compared to mRNA- and viral vector-based vaccines, SARS-CoV-2 protein subunit-based vaccines are easy to produce at a large-scale, and thus, can be beneficial to meet the high global demand of vaccines. Most of the protein subunit-based vaccines target spike RBD as it is the principal target of neutralizing antibodies. The benefits of adjuvants have enabled RBD-based vaccines to overcome their relatively poor immunogenicity. By activating pattern recognition receptors (PRRs) of the innate immune system, adjuvants can significantly increase the immunogenicity of vaccine antigens.
In the current study, the scientists have screened several PRR agonists and identified that toll-like receptor 9 (TLR9) agonists CpG oligodeoxynucleotides formulated with aluminum hydroxide and RBD can significantly increase the immunogenicity of RBD in young and old mice.
Study design
To identify the optimal combination of RBD antigen and adjuvants, the scientists used age-specific in vivo mouse models and in vitro human models. For the human experiments, peripheral blood samples were collected from healthy young adults and older adults.
For the animal experiments, several PRR agonists were formulated with aluminum hydroxide to be used as adjuvants. Mice were injected with recombinant RBD protein with or without adjuvants.
Separately, the mRNA-based Pfizer/BioNTech COVID-19 vaccine was also used to immunize the mice to compare its efficacy with adjuvanted RBD-based vaccine. After 2 weeks of immunization, blood samples were collected and levels of anti-spike and anti-RBD binding antibodies and neutralizing antibodies were measured.
Important observations
By screening several PRR agonist and aluminum hydroxide formulations, the scientists observed that the TLR9 agonist/aluminum hydroxide formation (AH:CpG formulation) has the highest ability to induce anti-RBD neutralizing antibody titers and inhibit RBD – angiotensin-converting enzyme 2 (ACE2) interaction in young mice.
By immunizing aged mice with AH:CpG-adjuvanted RBD vaccine, they observed significantly higher anti-RBD neutralizing antibody titers and inhibition of RBD – ACE2 interaction compared to the vaccine adjuvanted with a conventional adjuvant AS01B. However, compared to young mice, aged mice showed a lower immune response to the AH:CpG-adjuvanted RBD vaccine.
To improve vaccine-induced immunity in aged mice, they administered a second booster dose 2 weeks after the last immunization. This led to the induction of strong anti-RBD immunity in aged mice, which was comparable to that in young mice. Furthermore, AH:CpG-adjuvanted RBD vaccine showed high potency in inducing interferon response in immunized mice.
To investigate the protective ability of AH:CpG-adjuvanted RBD vaccine, they infected the experimental mice with SARS-CoV-2 6 weeks after immunization with the 2nd booster dose. The observations revealed that compared to the non-adjuvanted vaccine, AH:CpG-adjuvanted RBD vaccine provides higher protection against weight loss and lung pathologies.
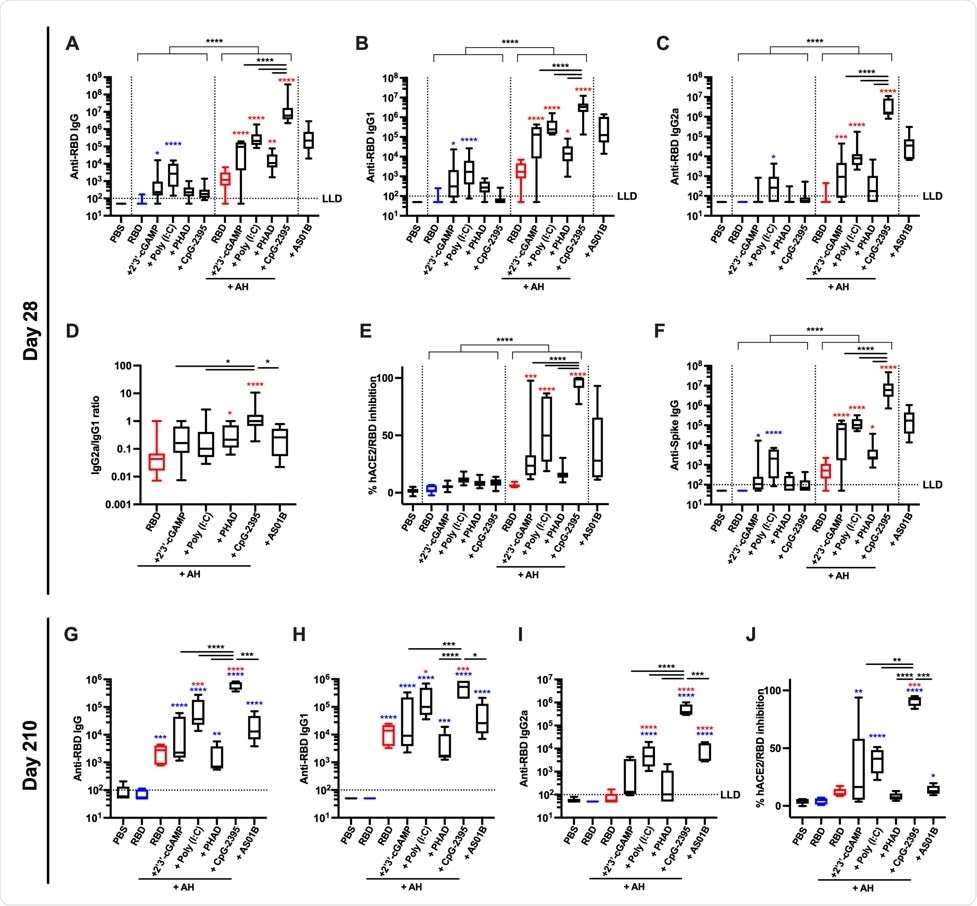
RBD formulated with AH:CpG induces robust production of anti-RBD neutralizing antibodies in young adult mice Young adult, 3-month-old BALB/c mice were immunized IM on Days 0 and 14 with 10 µg of monomeric SARS-CoV-2 RBD protein with indicated adjuvants. Each PRR agonist was administered alone or formulated with aluminum hydroxide (AH). (A–F) Serum samples were collected on Day 28, and (A) Anti-RBD IgG, (B) IgG1, (C) IgG2a, (D) IgG2a/IgG1 ratio, (E) hACE2/RBD inhibition rate, and (F) anti-Spike IgG were assessed. N=10 per group. Data were combined from two individual experiments. (G–J) Serum samples were collected on Day 210, and (G) Anti-RBD IgG, (H) IgG1, (I) IgG2a and (J) hACE2/RBD inhibition rate were assessed. N=5 per group. Data were analyzed by two-way (A–C, E–F) (AH and PRR agonist) or one-way (D, G–J) ANOVAs followed by post-hoc Tukey's test for multiple comparisons. *P <0.05, **P <0.01, ***P <0.001, **** P <0.0001. Blue and red colored asterisks respectively indicate comparisons to RBD and AH adjuvanted RBD groups. Box-and-whisker plots represent the minimum, first quartile, median, third quartile, and maximum value. LLD, lower limit of detection.
By comparing immune responses in mice immunized with AH:CpG-adjuvanted RBD vaccine or mRNA-based Pfizer/BioNTech COVID-19 vaccine, they observed that anti-RBD, anti-spike, and neutralizing antibody titers induced by AH:CpG-adjuvanted RBD vaccine is comparable to or greater than that induced by the mRNA-based vaccine.
Regarding SARS-CoV-2 variants, AH:CpG-adjuvanted RBD vaccine showed a 3.2-fold lower neutralizing antibody titer against the B.1.351 variant, whereas a 6-fold reduced titer against the variant was observed for mRNA-based COVID-19 vaccine.
To determine the mode of action of AH:CpG-adjuvanted RBD vaccine, they collected draining lymph nodes from mice injected with either CpG or AH:CpG. The findings revealed that both CpG and AH:CpG induce similar levels of lymph node expansion in young and aged mice. Moreover, comparable levels of cytokines, chemokines, and type I interferon immune responses were observed in mice treated with CpG or AH:CpG. These findings indicate that both CpG and AH:CpG are capable of activating the innate immune system in young and older mice to increase RBD immunogenicity.
Study significance
The study findings reveal that AH:CpG formulations can act as potent adjuvants to significantly improve the efficacy of RBD vaccines in both young and older mice.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources