Treatment options have been limited in the ongoing coronavirus disease 2019 (COVID-19) pandemic. Earlier optimism regarding immunomodulatory drugs such as azithromycin (AZM) and hydroxychloroquine (HCQ) seemed to be undermined by results of large interventional trials.
Using computational modeling, the use of weight-adjusted HCQ and AZM appears to be associated with a more than 100% increase in survival, without a clear correlation with ECG abnormalities.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Study details
In this study, based on a subset of critically ill COVID-19 patients, consisting of patients who required intubation and IMV, data from the medical records were analyzed using several novel methods. This included not only the vital signs and laboratory values but the therapeutic methods.
The study was carried out on patients at Saint Barnabas Medical Center, New Jersey, with just over 1% having been clinically diagnosed to have COVID-19. Of the 255 patients, almost 80% died during the study period. Seven patients were transferred to another hospital on the ventilator, mostly after day 40 of hospitalization.
Parameters were broadly comparable between survivors and non-survivors, except that all patients with an active malignancy, dementia, chronic obstructive pulmonary disease, and stroke failed to survive. However, sex, race, presentation severity, and blood type had no association with survival chances.
A pre-print version of the research paper is available on the medRxiv* server. A preprint is a version of a scholarly or scientific paper that precedes formal peer review and publication in a peer-reviewed scholarly or scientific journal.
Laboratory markers
Laboratory markers of inflammation, such as Ferritin, D-dimer, Lactate Dehydrogenase (LDH), and C-reactive protein (CRP), were above average in almost every patient (96%). While all parameters, except the LDH, were equivalent in survivors and non-survivors, three patients had D-dimer values above 69,000 ng/mL. LDH values were higher in non-survivors by almost 30%.
The increase in these parameters over time was characteristically steeper in patients who did not survive.
Clinical complications
More than three in four non-survivors developed acute kidney injury (AKI), of which a tenth received renal replacement therapy (RRT). Of this latter group, a fifth survived.
Almost 60% of patients were intubated within three days of hospitalization. The time to intubation did not predict survival, but intubation beyond day 15 was associated with survival in only 1 of 16 patients.
More than 90% of the patients in this cohort had high blood glucose levels above 140 mg/dL, peak at >200 mg/dL, without corticosteroid therapy. Although none were known to be diabetics, most probably had impaired glucose tolerance before they acquired SARS-CoV-2.
This prevalence is higher than in most other studies, probably because the researchers looked actively for hyperglycemia
Obesity
While half of the patients were obese, and 30% were overweight, the older patients were significantly heavier. That is, 74% of those above 60 were obese, vs 37% of those below this age.
The mean body weight was approximately 90 kg, but unlike most antibiotic clinical trials, the range of body weight was extensive. The heaviest patient thus weighed approximately seven times more than the lightest.
Notably, blood glucose levels or obesity did not predict a good clinical outcome.
Therapeutic drugs
The chief therapeutic classes included steroids, tocilizumab, convalescent plasma, hydroxychloroquine, and azithromycin.
Corticosteroids, when given at 6 mg or more, reduced the mortality risk 1.4 times. Meanwhile, the interleukin-6 receptor blocker) tocilizumab had two-fold lower mortality.
Convalescent plasma (CP) was used only from week 4, in a fifth of the patients, mostly younger than those who did not receive it. The survival of the group which received CP was almost doubled from CP non-users.
HCQ was used in 94% of patients within 48 hours of emergency room arrival, while >55% received 2,000-3,000 mg, cumulatively. Of this number, approximately 63% also received AZM. This combination fell out of favor over the study period based on external recommendations.
Effect of HCQ/AZM on mortality
With every log increase in the cumulative dose of HCQ, the mortality rate fell by 1.12 times, such that at 3 g HCQ, survival odds rose by 2.5 times.
When given together with AZM, the benefit was still more significant. Chances of survival increased further. Among those who received both > 3g HCQ and >1g AZM, almost half survived, compared to one in seven (16%) among patients who received one of these drugs at the same dosages.
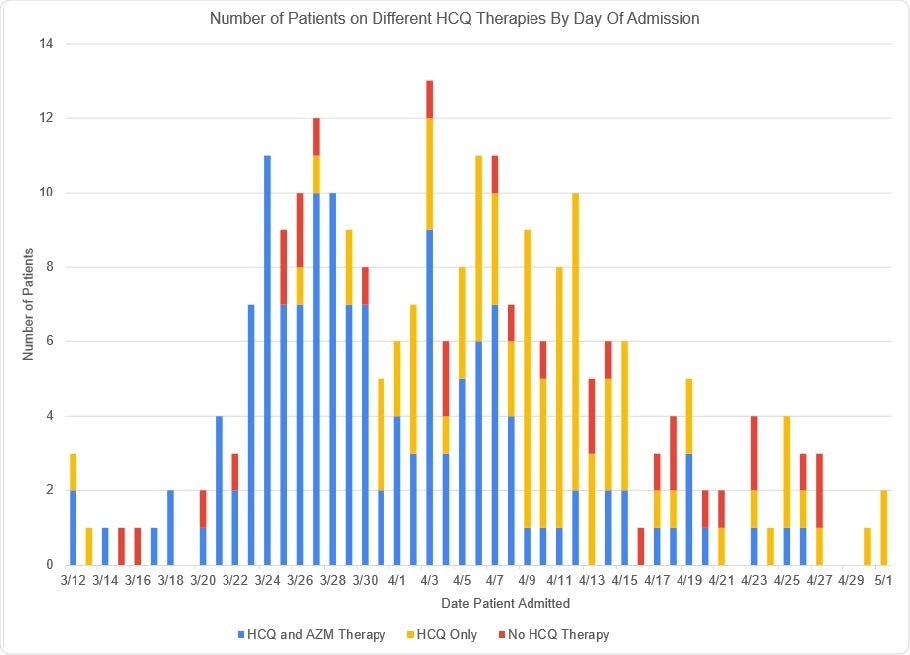
Number of patients by Date of Admission and breakdown by treatment with HCQ/AZM, HCQ alone or no HCQ therapy. Shown are the number of patients in the Cohort by admission date, from March 12 – May 1, 2020. HCQ therapy for each patient is demonstrated by use of color. Blue means the patient received HCQ and AZM therapy together, Gold, HCQ therapy without AZM, and Red, the patient did not receive HCQ.
This means a 32% absolute difference in survival, or a relative improvement in survival odds of 200%, with the combination of HCQ/AZM at this dosage. This far exceeds the survival benefit cited in any study of any intervention so far.
When HCQ/AZM was given at lower dosages, the risk of death was over three times higher relative to the above combination and dosage regimen.
When the cohort was divided into patients who received >3g HCQ/>1g AZM and those who did not, overall, the absolute chances of survival were 23% higher for the first group. The 17% survival in the second group would have increased to 39% with the former treatment, predicted the researchers.
This indicates that treatment with >3g HCQ/>1g AZM was associated with a more than 130% increase in survival rate compared to any other standard therapy.
Weight-adjusted cumulative dosage
The researchers also found that when adjusted for weight, the cumulative dose would have a still greater effect. In fact, the average treatment effect (difference in mean survival, in this case) shows a steep increase between 40-50 mg/kg to peak at 46% for a dose of 82 mg/kg.
Thus, patients receiving HCQ above 80 mg/kg of HCQ with >1g AZM had 14 times higher survival odds compared to those who did not. If HCQ dosage was fixed at >3g, the odds of survival were 7 times higher, or less than half of that achieved with the weight-adjusted cumulative dosage.
“The fact that weight-adjusted cumulative dose has an even greater effect on survival than cumulative HCQ dose is strong confirmation of the causal relationship between this treatment and improvement in survival rate.”
Age was another major factor since those older than 60 were five times more likely to succumb than younger patients. Hyperlipidemia was the single comorbidity linked to approximately four times higher odds of death.
Interestingly, there was no correlation between the cumulative dose of HCQ (or AZM) and the occurrence of QTc prolongation. In fact, the QT interval began to fall during the period when the cumulative dose of HCQ increased. None of the patients showed torsades de pointes.
What are the implications?
These findings indicate that a steeply rising ferritin, D-dimer and LDH over time predict poor survival, the rate of rise being several times greater for non-survivors. This should be validated to help provide a better prognosis for COVID-19 patients.
The extensive range of obesity among critically ill patients indicates that weight-adjusted dosage is critical in achieving the correct therapeutic levels. Moreover, AZM is an independent contributor to improved survival.
Most importantly, this is the first clinical study to demonstrate the remarkable benefit of using cumulative doses of HCQ>3g/AZM>1g, compared to those not treated with this combination.
Why did such a large effect miss observation? For one thing, HCQ produces its benefit by cumulative effects on the target cells, which is weight-dependent. The failure to treat patients with weight-adjusted doses leads to ineffective treatment and outcomes biased towards lighter patients.
HCQ is both safe and tolerable at higher doses, as shown in studies of rheumatoid arthritis or lupus. Such high doses for such long durations have not been used to treat COVID-19.
The earlier studies claiming prolongation of the QTc duration with HCQ in COVID-19 treatment are shown to be flawed. Indeed, available data suggests that this finding is due to the underlying illness itself.
The investigators also point out: “On April 24, 2020, the FDA issued a warning about the possible effects of low HCQ on QTc interval (47). Since 2010, the FDA has approved over 150 clinical trials, which include HCQ treatment. The FDA did and does not require monitoring for cardiotoxicity. In each of these trials, the total HCQ dose and expected tissue levels are markedly higher than used or seen in Covid patients. This discrepancy lacks logic or explanation.”
In this startling study, the investigators carefully re-examined the data, showing that among critically ill COVID-19 patients on IMV, less than 4% “walk out of hospital.” In contrast, the survival benefit of combined HCQ/AZM at a cumulative dosage of >80 mg/kg and >1g, respectively, is shown to be both clear and significant.
The safety at such doses is obvious, since survival is increased by almost 130% in this very high-risk population. Moreover, it appears that AZM is an important component of this therapy in terms of mortality reduction.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.