The search for safe and effective drugs to counter the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been on ever since the first wave showed the devastating effects of the virus on a significant minority of those infected.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Dual protease targets
The SARS-CoV-2 virus engages its host cell via several different pathways. The best known involves the angiotensin-converting enzyme 2 (ACE2), the receptor on the host cell, to which the viral spike protein attaches itself. This triggers a massive conformational change that causes the viral membrane to fuse with the host cell.
This fusion facilitates viral entry into the host cell cytoplasm via endosomal trafficking. However, for the spike protein to do this, it must be primed by a host protease enzyme, such as TMPRSS2, or by cathepsins, during the process of endocytosis.
Cathepsin is mostly localized in the late endosomes or lysosomes, with optimal activity at an acidic pH, which has led to the postulate that viral entry is via the endolysosomal compartment. Conversely, TMPRSS2 is a cell surface enzyme and functions over a range of pH levels, indicating that it cleaves the spike protein on viral particles at the plasma membrane, facilitating viral entry.
Early in the pandemic, it was discovered that the cathepsin inhibitor E-64 and the TMPRSS2 inhibitor camostat mesylate blocked SARS-CoV-2 infection. However, their low binding affinity for the proteases rendered them ineligible for clinical trials.
Another TMPRSS2 inhibitor, nafamostat mesylate, has a high nanomolar affinity for the enzyme.
Apilimod inhibits late endosomes
Meanwhile, a drug called apilimod was shown to inhibit another enzyme involved in endosomal trafficking, preventing viral entry, though its mechanism of action is not clear.
This enzyme, called PIKfyve kinase, is required to generate a key phosphate-containing molecule that regulates many proteins participating in late endosomal viral trafficking. Inhibition of this enzyme leads to the prevention of infection with Ebola, Marburg, and SARS-CoV-2, as has been demonstrated in earlier studies.
PIKfyve kinase may regulate cathepsin levels in the endosome because all cells in which E-64 inhibited viral entry by blocking cathepsin-mediated priming of the spike were also sensitive to the PIKfyve kinase inhibitor apilimod, while those which were resistant to the first also failed to show inhibition with the second.
Synergistic combination of inhibitors
SARS-CoV-2 infection is mediated by two protease pathways that complement each other, one depending on cathepsin and the other on TMPRSS2 to prime the spike protein for fusion. Cell lines which expressed TMPRSS2 but did not respond to cathepsin inhibition were completely sensitive to TMPRSS2 inhibitors.
The current study tested different cells with varying levels of these proteases, using different combinations of protease inhibitors with apilimod, to assess how each inhibited a pseudovirus expressing the SARS-CoV-2 spike protein.
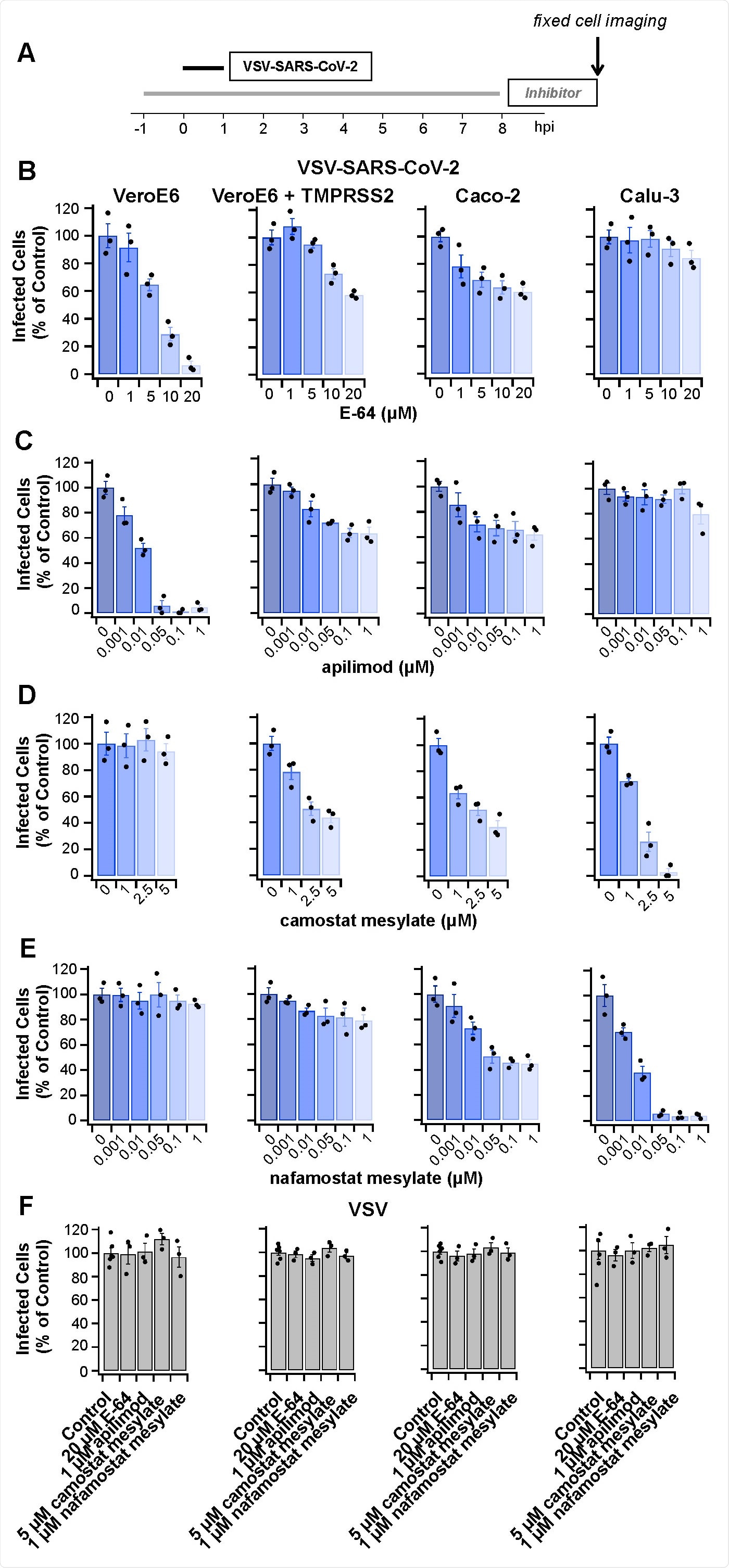
Protease inhibitors E-64, apilimod, camostat mesylate or nafamostat mesylate prevent infection by VSV-SARS-CoV-2 but not by VSV. (A) Schematic of infectivity assay for cells pretreated for 1 h or not with the inhibitors, subsequently infected with VSV-SARS-CoV-2 for 1 h in the presence or absence of inhibitors. The cells were incubated for another 7 h in the presence or absence of inhibitors and then fixed; the percentage of cells expressing eGFP measured by spinning disc confocal microscopy. (B-F) Quantification of the number of infected cells from three independent experiments, each determined from 5 fields of view containing 80-200 cells per experiment (error bars show SEM) for the indicated cell types. Infected Vero (B) or Vero + TMPRSS2 cells C) were analyzed 8 hpi using 0.5 µg/mL VSV-SARS-CoV-2 RNA. Infected Caco-2 (D) or Calu-3 cells (E) were analyzed 8 hpi using 5 µg/mL VSV-SARS-CoV-2 RNA. Cells infected with 0.075 µg/mL VSV RNA (F) were analyzed 6 hpi. In each case, these virus concentrations and conditions of infection corresponded to an MOI of ~ 0.5.
At the concentrations used, cytotoxicity was not observed.
Combinations of protease inhibitors with apilimod were found to show a striking increase in the efficacy of the inhibition, indicating synergism rather than simple additive effects.
The use of apilimod and camostat mesylate reduced the 50% effective concentration (EC50) of the latter two-fold against SARS-CoV-2, while in cells lacking cathepsin, the EC50 decreased five-fold. A combination of apilimod and nafamostat mesylate reduced the EC50 20-fold.
With higher concentrations of nafamostat mesylate, the combination led to a ten-fold reduction in the EC50 of apilimod. The combination was equally synergistic against pseudoviruses expressing the D614G spike variant, which is known to be more infective.
This strong synergism was caused by the inhibition of both TMPRSS2 and the endosomal enzyme PIKfyve kinase, by nafamostat mesylate and apilimod, respectively, both at nanomolar concentrations.
A control virus that does not require any of these enzymes for entry was able to infect these cell lines, as expected.
The combination of inhibitors of these two enzymes also prevented SARS-CoV-2 infection, with equally strong synergism.
What are the implications?
Apilimod blocks late endosomal viral traffic and also inhibits cathepsin-mediated viral entry into the cells via late endosomes. The TMPRSS2 inhibitors prevent TMPRSS2-mediated viral infection.
The combination of these inhibitors, acting against two different viral entry factors, generated intense synergistic activity against SARS-CoV-2 entry into target host cells, compared to the use of either in isolation. This is in contrast to the additive effects seen with the combined use of cathepsin inhibitors with TMPRSS2 inhibitors to block infection by SARS-CoV-2.
Such inhibition was present against both a pseudovirus expressing SARS-CoV-2 spike protein and the SARS-CoV-2 virus itself.
Modified versions of these inhibitors with increased oral bioavailability are currently undergoing clinical trials, being used alone to treat various conditions, including COVID-19. Intravenous formulations of nafamostat mesylate, with greater solubility, are being tested for COVID-19 therapy.
It is supposed, based on the synergism observed in this study, that using PIKfyve and TMPRSS2 inhibitors in combination could increase the efficiency of viral inhibition by 5-10-fold in the host cells, marking an advance in COVID-19 treatment.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources