Do T-cells play a protective immune role, or are they responsible for severe COVID-19 illness? An international team of scientists sought to answer this question and determine the cause behind the disease pathology.
T-cells are essential in the immune system for controlling acute respiratory infections. But excessive T-cell activation causes more harm than good by increasing infection-associated organ damage.
The team’s results showed that patients with severe COVID-19 infection tend to have an increased amount of a specific population of T-cells known as CD16+ T-cells. These T-cells have cytotoxic properties that damage the body, causing the activation and release of chemokines and lung injury. As a result, the findings suggest that complement-mediated formation of activated CD16+ T-cells contributes to the pathology of severe COVID-19 disease.
Taken together, particularly severe COVID-19 leads to an elevation of activated CD16+ T cells that link the elevated complement cascade via TCR-independent cytotoxic T cell functionality to endothelial damage and patient survival, thereby establishing a novel immunopathological link between the innate immune system, the adaptive immune compartment, and endothelial injury, which might constitute an important molecular axis explaining the vast spectrum of organ damage observed in COVID-19.”
The study “Complement activation induces excessive T cell cytotoxicity in severe COVID-19” is available as a preprint on the medRxiv* server.
The study
The researchers collected blood samples from patients with SARS-CoV-2 along with healthy controls. They performed mass cytometry and scRNA-seq on T-cells from these patients.
They identified a highly activated T cell population expressing CD16+ across three T cell compartments — CD4+, CD8+ TCRab+, and TCRgd+ T cells. The activated CD16+ population in patients with severe COVID-19 infection was absent in other acute or chronic infections.
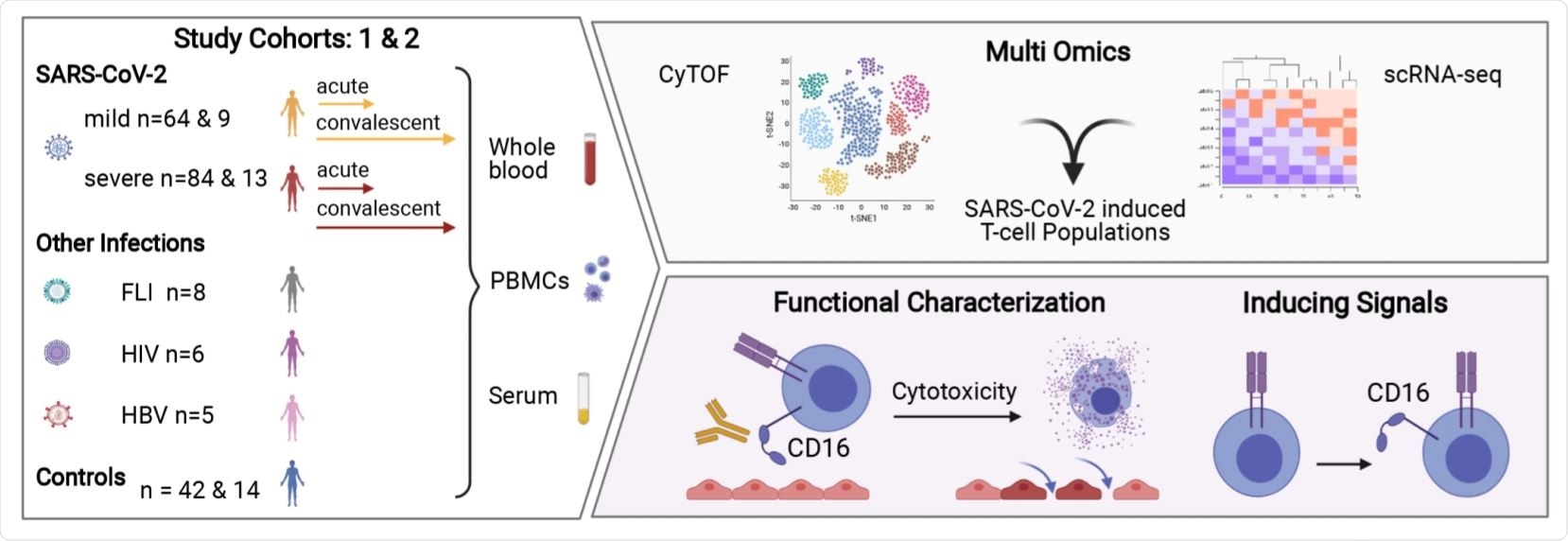
Overview of the Study. Image Credit: https://www.medrxiv.org/content/10.1101/2021.06.08.21258481v1.full.pdf

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
C3a induces activation of cytotoxic T cells
The population of cells expressed a cytotoxic program independently of T-cell receptor stimulation. Further analysis identified the complement split product C3a as an inducing signal. In patients with severe COVID-19 disease, a generation of C3a resulted in excess CD16+ T-cell activation.
“Thus, in severe COVID-19 T cell activation and functionality is altered due to an imbalanced, aged, and C3a-rich environment.”
CD16+ T-cells maintain cytotoxicity throughout infection
An increased C3a generation in patients with severe COVID-19 infection promoted differentiation of CD16 expressing, highly cytotoxic CD4+ and CD8+ T cells. Additionally, CD16+, CD4+, and CD8+ T cells expressed high chemokine receptor levels, including CXCR3 and CCR6. Functional assays showed these T cells damaged the endothelium.
The levels of complement signals were also higher in patients with severe COVID-19 disease. Compared to blood samples of patients with pneumonia but not SARS-CoV-2, there were more FCGR3A+ T cells with high cytotoxic and degranulation potential.
They next looked to find out how long cytotoxicity lasted. TCR-sequencing was used to track T-cell clones. Cytotoxicity in severe disease persisted for up to 6 months with CD16+ T cells expressing high PRF1, GZMB, LAMP1, and STX11 levels. Results showed the CD16+ T cells have a differentiated phenotype similar during COVID-19 convalescence, indicating they persist in the convalescent phase and maintain their cytotoxic properties.
Study limitations
There were not enough convalescent samples available for scRNA-sequencing analysis. Because of this, results comparing the differences in clonal persistence and phenotype between mild and severe infection are limited. The researchers expressed the need for more extensive studies to confirm the increasing persistence for late differentiated, CD16 expressing, and highly cytotoxic T lymphocytes.
The low number of convalescent samples also made it difficult to investigate how this specific T cell population correlated with patient recovery.
The researchers suggest future work studying the pathology of severe COVID-19 illness should consider applying the C3 inhibitor AMY-101 in patients with COVID-19 and ARDS to see whether it would prevent the differentiation of this T cell population. If successful, a C3 inhibitor could help with treated COVID-19-induced endothelial cell injury and lymphocytic endotheliitis.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Georg P, et al. Complement activation induces excessive T cell cytotoxicity in severe COVID-19. medRxiv, 2021. doi: https://doi.org/10.1101/2021.06.08.21258481, https://www.medrxiv.org/content/10.1101/2021.06.08.21258481v1
- Peer reviewed and published scientific report.
Georg, Philipp, Rosario Astaburuaga-García, Lorenzo Bonaguro, Sophia Brumhard, Laura Michalick, Lena J. Lippert, Tomislav Kostevc, et al. 2021. “Complement Activation Induces Excessive T Cell Cytotoxicity in Severe COVID-19.” Cell, December. https://doi.org/10.1016/j.cell.2021.12.040. https://www.cell.com/cell/fulltext/S0092-8674(21)01562-2.