Researchers in the UK have reported on the relationship between humoral (antibody) responses and protection against infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) following immunization with AstraZeneca’s ChAdOx1 vaccine.
The SARS-CoV-2 virus is the agent responsible for the coronavirus disease 2019 (COVID-19) pandemic that continues to pose a threat to global public health and has now caused more than 3.92 million deaths worldwide.
The study analyzed data from a phase 2/3 vaccine efficacy trial conducted across 19 sites in the UK.
The team from the University of Oxford, NIHR Oxford Biomedical Centre, AstraZeneca PLC, Public Health England in Salisbury and the Oxford COVID Vaccine Trial Group say the analysis aimed to facilitate the development and licensure of future vaccines in situations where large vaccine efficacy trials are not feasible.
“The data can be used to extrapolate efficacy estimates for new vaccines where large efficacy trials cannot be conducted,” writes Merryn Voysey and colleagues.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Rapid authorization of new vaccines could help meet demand
Since the COVID-19 outbreak first began in late December 2019, six vaccines designed to protect against SARS-CoV-2 infection have been approved by the World Health Organization.
Real-world studies of vaccination rollout programs have demonstrated the vaccine’s high efficacy in protecting against severe disease, hospitalization, and death, as well as asymptomatic infection and within-household transmission.
Despite mass production efforts, the supply of COVID-19 vaccines remains limited in some countries and the rapid authorization of new vaccines could help meet global demand, says Voysey and colleagues.
However, “as more countries implement vaccine programs, it will become increasingly difficult to conduct clinical efficacy studies of new vaccines,” they write. “Understanding the relationship between immune responses to vaccines and protection against clinical outcomes is urgently needed to speed vaccine development.”
Knowledge about “correlates of protection” could facilitate rapid licensure of new vaccines
Knowledge about the immune measures that are statistically associated with protection against disease – “correlates of protection” – may permit the authorization of new vaccines based on immunogenicity and safety data alone when large efficacy trials are not feasible, say the researchers.
Both the binding and neutralizing antibodies that are generated following SARS-CoV-2 infection or vaccination are considered potential correlates of protection. Levels of these antibodies have also been shown to be correlated with each other.
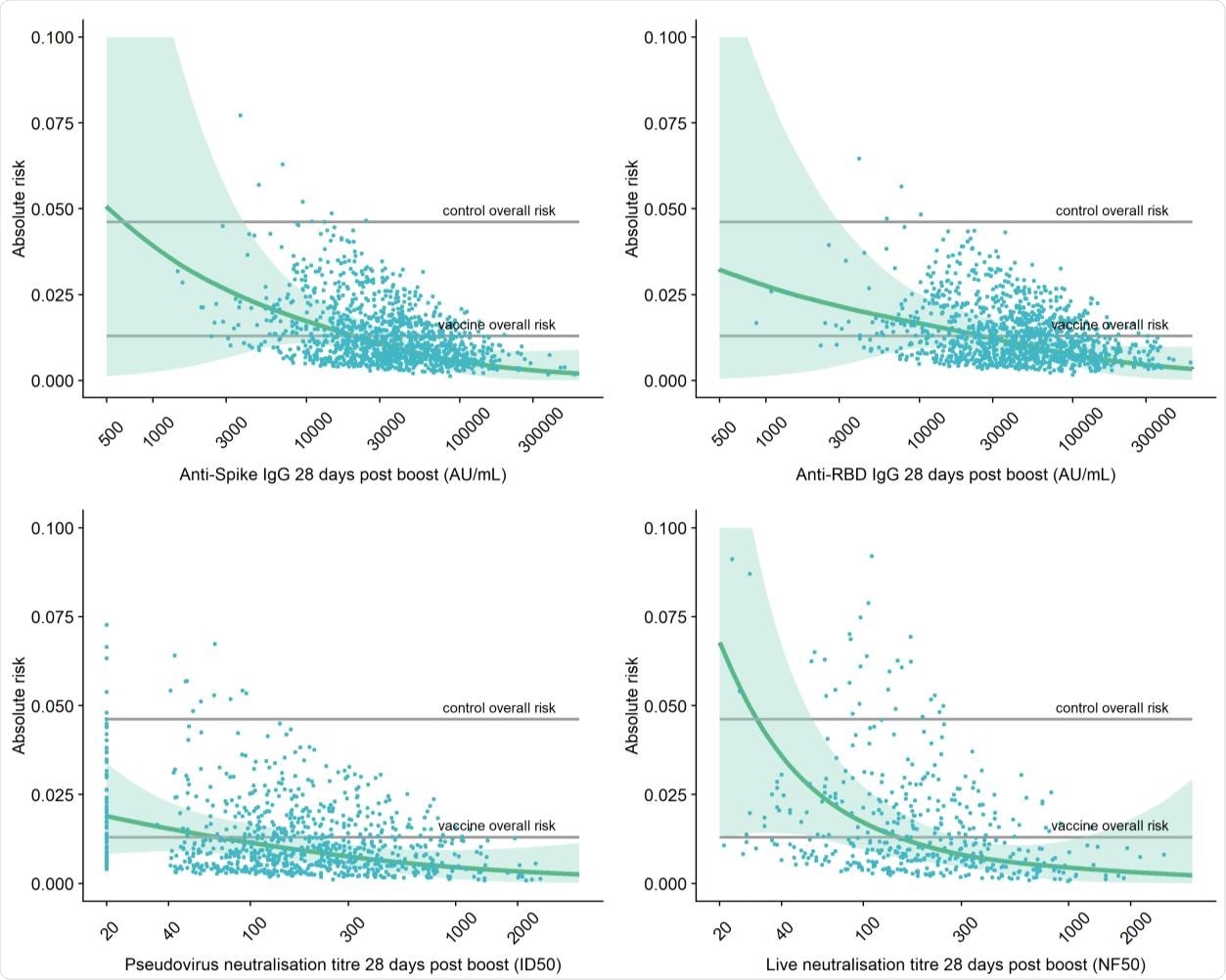
Adjusted risk of primary symptomatic COVID-19 as a function of immune markers measured 28 days post second dose.
The ChAdOx1 vaccine developed by AstraZeneca, an adenovirus-based vaccine containing the full-length SARS-CoV-2 spike protein, is now being administered on a mass scale globally. The viral spike protein is the main structure the virus uses to bind to and infect host cells.
The receptor-binding domain (RBD) of spike mediates the initial stage of the infection process when it binds to the human host cell receptor angiotensin-converting enzyme 2 (ACE2). Thus, the spike RBD is the main target of neutralizing antibodies following SARS-CoV-2 infection or vaccination.
“We previously showed that estimates of vaccine efficacy against symptomatic COVID-19 infection were higher in subgroups with higher pseudovirus neutralization antibody titers, or increased anti-spike immunoglobulin G (IgG) in vaccine clinical trials of ChAdOx1,” writes the team.
What did the current study involve?
The team analyzed data from a phase 2/3 randomized efficacy trial of the ChAdOx1 vaccine conducted across 19 sites in the UK to determine the antibody levels that are associated with protection against SARS-CoV-2 infection.
“Here, we report the relationship between the humoral immune responses to vaccination and protection afforded by this vaccine to facilitate further vaccine development,” says Voysey and colleagues.
Infected and uninfected participants had their levels of anti-spike IgG and anti-RBD IgG, as well as levels of neutralizing antibodies against pseudovirus and live virus measured 28 days following receipt of a second vaccine dose.
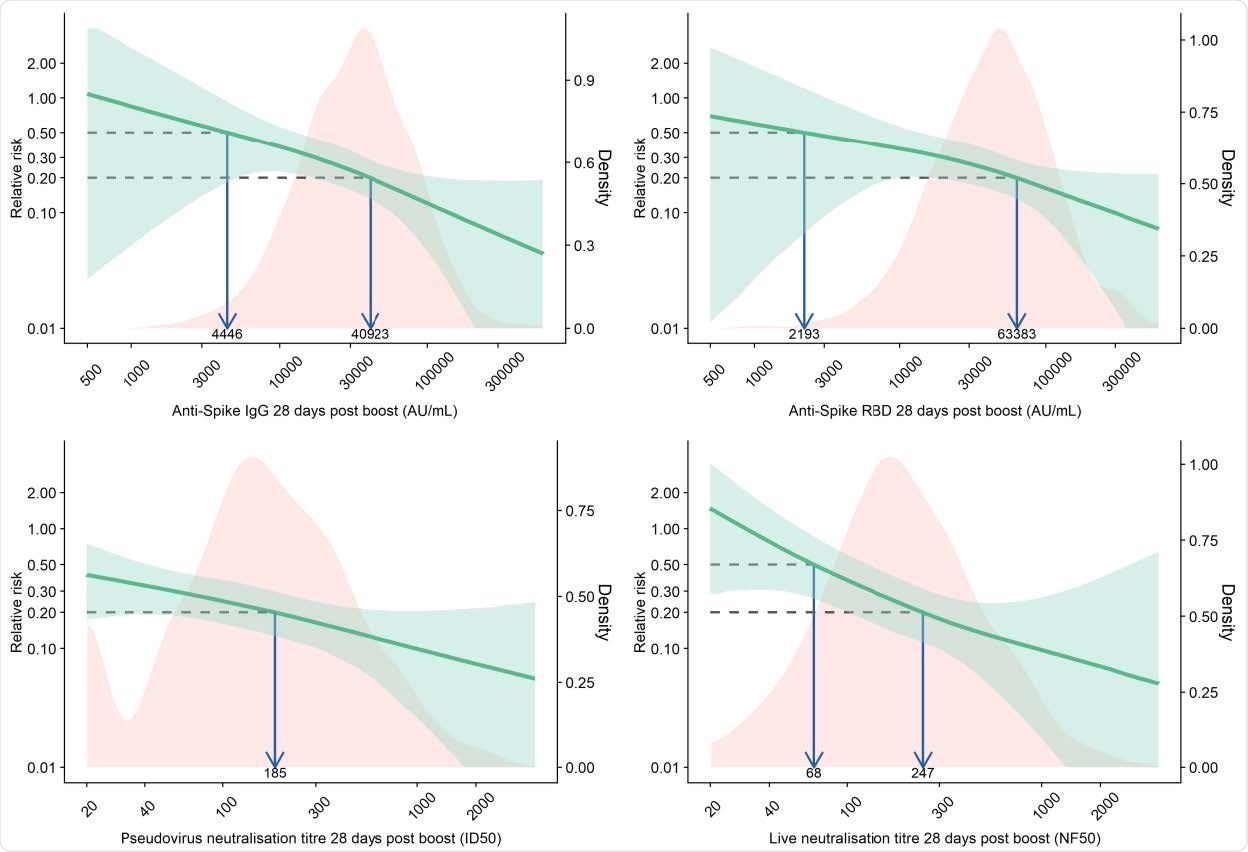
Relative risk of primary symptomatic COVID-19 among vaccine recipients compared with MenACWY control arm participants as a function of immune markers measured at day 28 post-second dose
What did they find?
Higher levels of all four immune markers tested were correlated with a reduced risk of symptomatic infection.
Levels of anti-spike and anti-RBD IgG were highly correlated with each other and levels of pseudovirus and live virus neutralizing antibodies were modestly correlated. Anti-spike IgG titers were also correlated with pseudovirus neutralization titers and live virus neutralization titers.
When primary symptomatic COVID-19 cases were classified according to the presence of shortness of breath, a similar trend was observed, with higher levels of all immune markers associated with a lower risk of symptomatic infection.
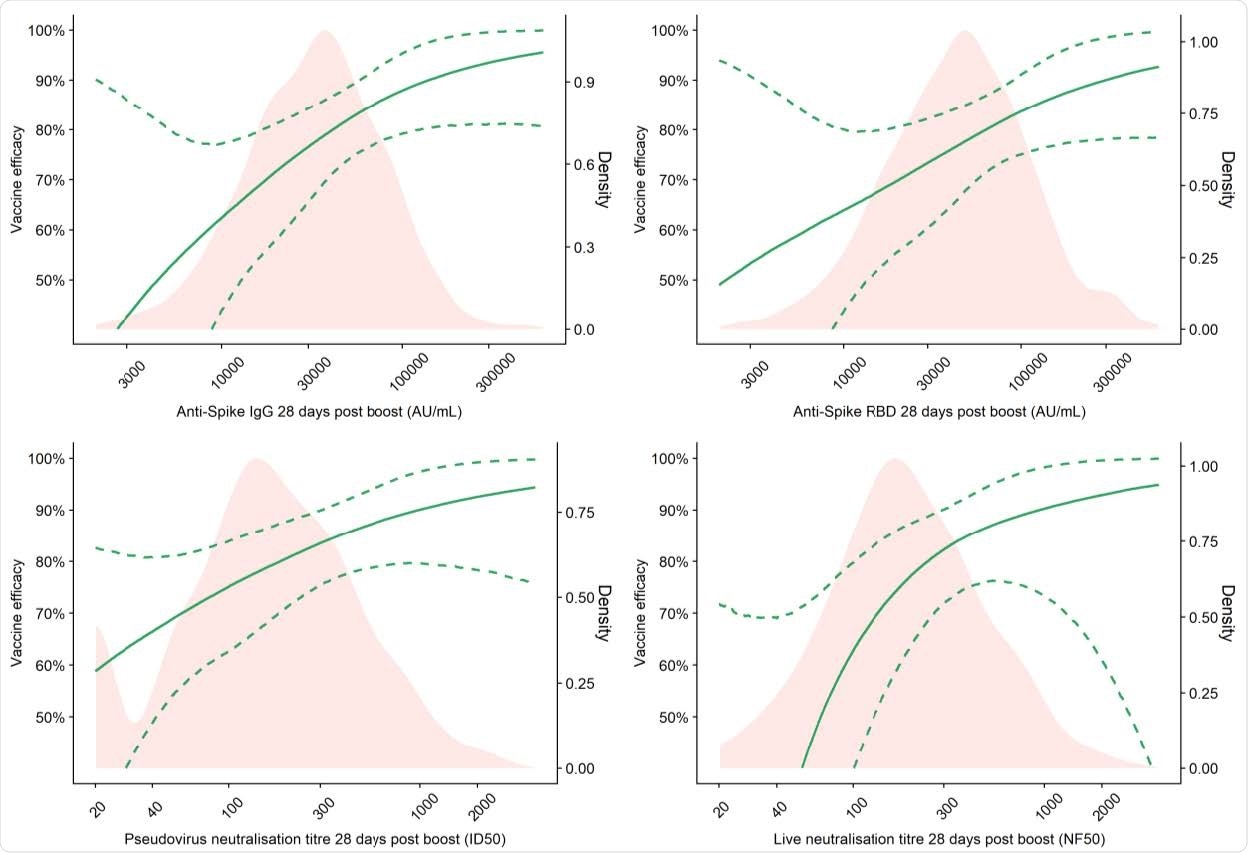
Vaccine efficacy against primary symptomatic COVID-19 as a function of immune markers measured at day 28 post-second dose
Among those with three or more COVID-19 symptoms, higher titers of pseudovirus and live virus neutralizing antibodies were associated with a lower risk of infection.
Vaccine efficacy of 80% against primary symptomatic COVID-19 was achieved with an IgG level of 40,923 arbitrary units (AU)/mL for anti-spike and 63,383 Au/mL for anti-RBD. For pseudovirus and live virus-neutralizing antibody titers, a vaccine efficacy of 80% against symptomatic infection was achieved at neutralizing titers of 185 and 247, respectively.
By contrast, none of the serological values were correlated with protection against asymptomatic infection.
“This was unsurprising and is consistent with our interim combined vaccine efficacy analysis from UK and Brazil that vaccine efficacy against asymptomatic infection was 27.3% (−17.2 to 54.9),” writes the team.
What are the implications of the study?
The researchers say that protection against symptomatic COVID-19 is not absolute with any vaccine. The results presented here show there is no single threshold value for any of the assays investigated that indicates sterilizing immunity.
“Instead, the probability of infection decreases on average with higher immune responses,” they write.
“The correlates of vaccine efficacy reported here could be used to extrapolate efficacy to immunogenicity data for novel vaccines where clinical efficacy results are unlikely to be obtained,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Voysey M, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv, 2021. doi: https://doi.org/10.1101/2021.06.21.21258528, https://www.medrxiv.org/content/10.1101/2021.06.21.21258528v1
- Peer reviewed and published scientific report.
Feng, Shuo, Daniel J. Phillips, Thomas White, Homesh Sayal, Parvinder K. Aley, Sagida Bibi, Christina Dold, et al. 2021. “Correlates of Protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection.” Nature Medicine 27 (11): 2032–40. https://doi.org/10.1038/s41591-021-01540-1. https://www.nature.com/articles/s41591-021-01540-1.