Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent for Coronavirus Disease 2019 (COVID-19), emerged in late December 2019, leading to the ongoing worldwide pandemic.
With over 182 million reported infections worldwide, there has been ample opportunity for the virus to mutate, and numerous variants of concern (VOC) have emerged, including the B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617 (Delta), P.1 (Gamma), B.1.526 (Iota), and B.1.427/9 (Epsilon) variants.
The availability and distribution of vaccines are now widespread. However, the pace and geographic limitations in access to vaccines still engender an increase in infections and the requirement for effective therapies.
One promising treatment approach that has been extensively explored is the identification of SARS-CoV-2-neutralizing antibodies that can be useful as therapeutic or prophylactic agents for COVID-19. Antibodies that neutralize the SARS-CoV-2 virus mainly target the receptor-binding domain or the N-terminal domain (NTD). More than a dozen neutralizing NTD-directed antibodies have been structurally analyzed, and all of them target a single antigenic supersite in NTD.
A cryo-EM structure of an NTD-directed neutralizing antibody that forms a complex with SARS-CoV-2 spike protein
Researchers from the US recently reported the 3.7 Å resolution cryo-EM structure of an NTD-directed neutralizing antibody 5-7 that forms a complex with SARS-CoV-2 spike protein. They also presented the neutralization data against VOC and studied the recognized epitope. This antibody recognizes a site distinct from other neutralizing antibodies, and they insert a binding loop into an exposed hydrophobic pocket located between the 2 sheets of the NTD b-sandwich. This study is published on the bioRxiv* preprint server.
Interestingly, this exposed pocket has been previously identified as the binding site for hydrophobic molecules such as heme metabolites. However, this study observed that their presence does not significantly hinder recognition. Furthermore, thanks to its distinct binding, the antibody 5-7 also retains a distinct neutralization potency against SARS-CoV-2 variants of concern.
Results show that antibody 5-7 targets a conserved epitope on NTD, thus enabling more effective therapeutic strategies
Overall, the findings reveal a hydrophobic pocket in NTD proposed for immune evasion, which can be used by the human immune system for recognition. The structure of antibody 5-7 in complex with the spike protein of SARS-CoV-2 shows that potent neutralization is possible using antibodies that target NTD outside the site 1 supersite.
“Despite the clear structural uniqueness of antibody 5-7, prior experiments showed binding competition between 5-7 and supersite-directed antibodies.”
In vitro experiments show that supersite-directed antibodies neutralize by inhibiting the conformational changes required for viral fusion and not by blocking the recognition of the ACE2 receptor. According to the authors, the NTD conformation they observed in the antibody 5-7 complex structure is unique and seems to be incompatible with the binding of other NTD-directed antibodies. Although the hydrophobic pocket may play a role in SARS-CoV-2 biology, including interacting with a target protein-ligand, it remains unknown to date.
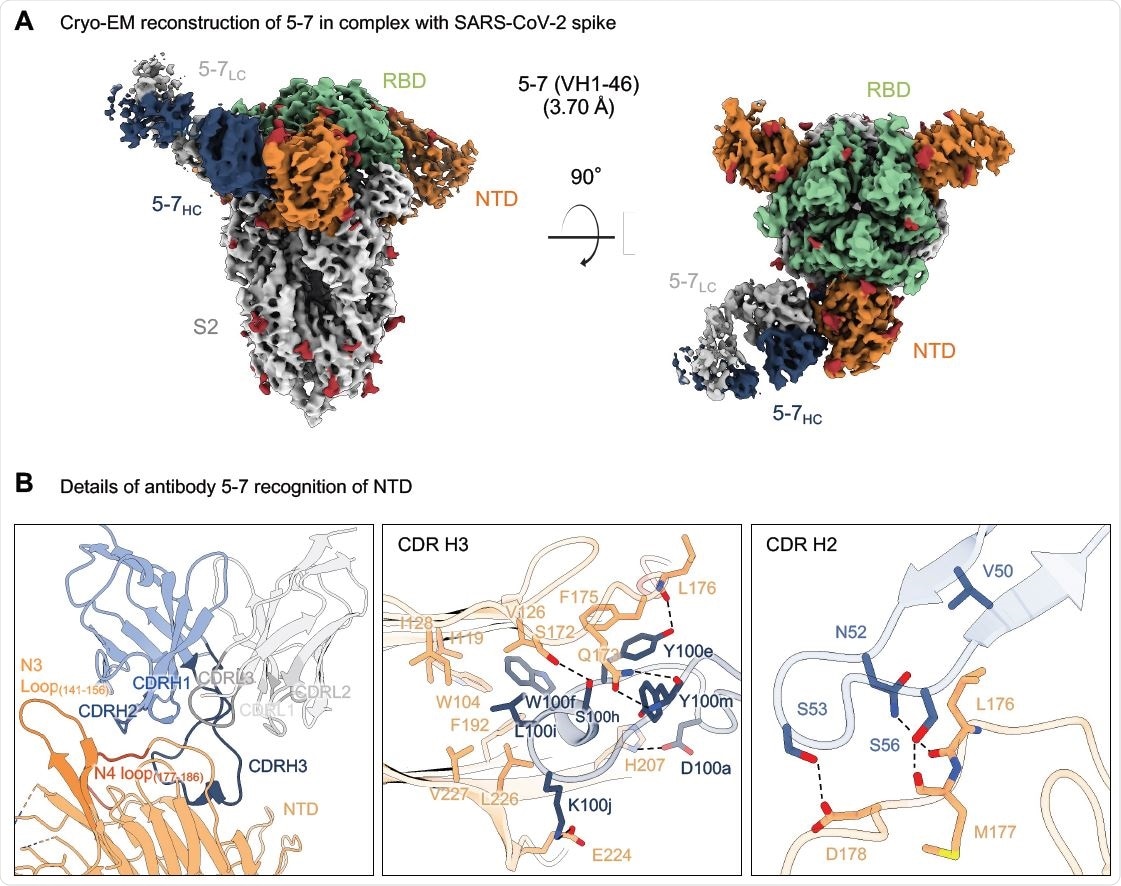
Antibody 5-7 targets a hydrophobic pocket in NTD (A) Cryo-EM reconstruction for spike complex with antibody 5-7 from two orthogonal views. NTD is shown in orange, RBD in green, glycans in red, antibody heavy chain in blue and light chain in gray. (B) Details of antibody 5-7 recognition of NTD showing the overall interface (left panel), recognition by CDR H3 (middle panel) and recognition by CDR H2 (right panel). CDR H1, H2, H3 are colored in shades of blue; CDR L1, L2, and L3 are colored in shades of gray. Nitrogen atoms are colored in blue, oxygen atoms in red; hydrogen bonds (distance <3.2 Å) are represented as dashed lines.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
One of the most attractive properties of antibody 5-7 is the partial tolerance of its neutralization to mutations in VOC. The study shows that this tolerance arises from its novel binding orientation, which results in a lack of overlap of 5-7 with VOC mutations.
Overall, the results show that antibody 5-7 targets a conserved epitope on NTD, thus increasing the number of known neutralizing epitopes. This also enables therapeutic strategies, including cocktail combinations or multispecifics that can target both NTD and RBD.
“Here we report the 3.7 Å resolution cryo-EM structure of a potent NTD-directed neutralizing antibody 5- 7, which recognizes a site distinct from other potently neutralizing antibodies, inserting a binding loop into an exposed hydrophobic pocket between the two sheets of the NTD b-sandwich.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Neutralizing antibody 5-7 defines a distinct site of vulnerability in SARS-CoV-2 spike N-terminal domain, Gabriele Cerutti, Yicheng Guo, Pengfei Wang, Manoj S. Nair, Yaoxing Huang, Jian Yu, Lihong Liu, Phinikoula S. Katsamba, Fabiana Bahna, Eswar R. Reddem, Peter D. Kwong, David D. Ho, Zizhang Sheng, Lawrence Shapiro, bioRxiv, 2021.06.29.450397; doi: https://doi.org/10.1101/2021.06.29.450397, https://www.biorxiv.org/content/10.1101/2021.06.29.450397v1
- Peer reviewed and published scientific report.
Cerutti, Gabriele, Yicheng Guo, Pengfei Wang, Manoj S. Nair, Maple Wang, Yaoxing Huang, Jian Yu, et al. 2021. “Neutralizing Antibody 5-7 Defines a Distinct Site of Vulnerability in SARS-CoV-2 Spike N-Terminal Domain.” Cell Reports 37 (5): 109928. https://doi.org/10.1016/j.celrep.2021.109928. https://www.cell.com/cell-reports/fulltext/S2211-1247(21)01401-7.