Researchers in the Netherlands have conducted a study showing that Janssen’s Ad26.COV2.S coronavirus disease 2019 (COVID‐19) vaccine was effective against variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among eight individuals.
However, while all variants of concern tested were susceptible to vaccine-induced neutralizing activity, the effect was reduced compared with that observed for the first variant of SARS CoV-2 (B.1 lineage).
Compared with vaccine-induced neutralizing activity against the B.1 virus, the reduction in neutralization was greater for the B.1.351 (Beta) and P.1 (Gamma) variants than that observed for the rapidly spreading B.1.617.2 (Delta) variant.
Mandy Jongeneelen and colleagues from Janssen Vaccines & Prevention in Leiden say that while the efficacy of the Janssen vaccine against B.1.617.2 is currently unknown, they suspect that just one dose will be sufficient to confer protection.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Janssens’ vaccine is currently being tested in clinical trials
The Ad26.COV2.S vaccine developed by Janssen is currently being tested in several clinical trials, and interim data on immunogenicity and efficacy have been reported.
The data have so far shown that the vaccine induces robust antibody and cellular immunity against the original SARS-CoV-2 isolate identified in Wuhan, China (Wuhan-Hu-1) and shows high efficacy (more than 80%) in preventing severe COVID-19.
The vaccine was also shown to completely protect against COVID-19-related hospitalization and death, including in South Africa, where more than 95% of individuals for whom sequence data was available were infected with the B.1.351 (Beta) variant of concern.
As of February 2021, the single-dose Janssen vaccine had been granted emergency use authorization or marketing authorization in more than 50 countries, and more than 19 million people have now received the vaccine globally.
Vaccine-induced neutralization of B.1351 and P.1 may be reduced
Jongeneelen and colleagues recently demonstrated that the neutralization activity was reduced against B.1.351 and P.1, by 5.0- fold and 3.3-fold, respectively, compared with activity against the Wuhan-Hu-1 variant.
However, non-neutralizing functions including cellular phagocytosis, complement deposition, natural killer cell activation and CD4 and CD8 T cell responses were comparable across the Wuhan-Hu-1, B.1.1.7 (Alpha), B.1.351 and P.1 variants.
As the B.1.617.2 lineage that first emerged in India continues to spread across the world rapidly, data on vaccine efficacy against this variant are urgently needed, says the team.
What did the researchers do?
Now, Jongeneelen and colleagues have tested sera from recipients of a single dose of the Janssen vaccine for neutralizing activity against several variants of concern, including B.1.617.2.
The study included eight participants (aged 47 to 91 years) from the phase 3 ENSEMBLE trial that began on September 23rd, 2020.
Sera were collected from the participants 71 days after they had received the vaccine.
What did the study find?
The team reports that all variants tested exhibited susceptibility to vaccine-induced neutralization.
Neutralizing antibody titers against the B.1.1.7 and B.1 variants were comparable.
However, neutralization titers against all other variants were reduced by 1.5- to 3.6-fold, compared with those observed for the B.1 strain.
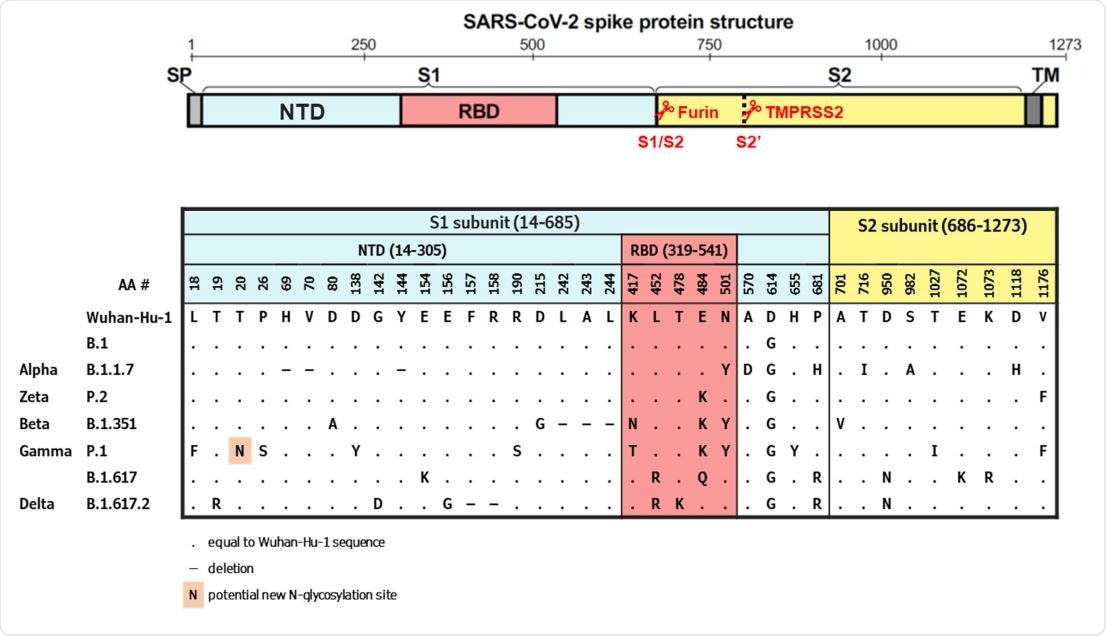
Spike protein schematic structure and alignment of substitutions in SARS‐CoV‐2 variants evaluated in this study. S, Spike protein; NTD, N‐terminal domain; RBD, receptor binding domain; SP, signaling peptide; TM, transmembrane domain; AA, amino‐acid.
The B.1.351 and P.1 variants exhibited the greatest reduction in neutralization activity (3.6- and 3.4-fold, respectively) compared with activity against B.1.
For the rapidly spreading B.1.617.2 variant, neutralizing titers were only reduced by 1.6-fold.
The team says these results are in accordance with those of recently published studies of individuals who had received the Moderna, Pfizer-BioNTech or Oxford-AstraZeneca vaccines.
Across all of these vaccines, greater reductions in neutralizing activity were observed for B.1.351 than for B.1.617.2.
What do the authors advise?
“At this point, no data on vaccine efficacy against the Delta variant is available, although real-world evidence studies have suggested that the Pfizer-BioNTech and Oxford-AstraZeneca vaccine are effective against this new variant,” says the team.
Jongeneelen and colleagues say that while the efficacy of the Janssen vaccine against B.1.617.2 is currently unknown, it may become available through the phase III ENSEMBLE trials later this year.
However, the researchers say they previously observed that the vaccine was highly effective at protecting against severe COVID-19 and fully protective against COVID-19-related hospitalization and death in South Africa. In that study, more than 95% of people with genomic sequencing data available were found to be infected with B.1.351, against which neutralizing titers were more severely impacted on day 21.
“This strongly suggests that the vaccine efficacy of a single dose of As26.COV2.S against the Delta variant will be preserved as well, either because lower neutralizing antibody titers are still sufficient to be protective or by the contribution non-neutralizing antibody functions and the strong cellular immune response that As26.COV2.S elicits,” they conclude.
Further Reading
Positive New Data for Johnson & Johnson Single-Shot COVID-19 Vaccine on Activity Against Delta Variant and Long-lasting Durability of Response - https://www.jnj.com/positive-new-data-for-johnson-johnson-single-shot-covid-19-vaccine-on-activity-against-delta-variant-and-long-lasting-durability-of-response

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.