Variants of concern and variants of interest of SARS-CoV-2 have been a distinctive feature of the coronavirus disease (COVID-19) pandemic during 2021, demonstrating the importance of viral sequencing in our epidemiological approach.
A variant of interest is “suspected” to be more infectious when compared to the initial strain, to escape the vaccine protection or result in more severe disease. Consequently, it can become a variant of interest if there is ample evidence that it actually has one or more of the aforementioned features.
A newly described SARS-CoV-2 lineage C.37 was recently designated as a variant of interest by the World Health Organization (WHO) on June 14th and denominated as the Lambda variant. Its presence was reported in more than 20 countries as of July 2021, and most sequences to date stem from South American countries – particularly for Chile, Argentina, Ecuador and Peru.
And albeit the Lambda variant is characterized by specific mutations in the SARS-CoV-2 spike glycoprotein, their exact impact of infectivity and potential escape to neutralizing antibodies are still unknown. The latter is particularly important in countries currently undergoing massive vaccination efforts.
Since one of such countries is Chile, a research group led by Dr. Mónica L. Acevedo from the University of Chile School Of Medicine aimed to appraise the impact of the Lambda variant on the neutralizing antibodies responses stemming from the inactivated virus vaccine CoronaVac (also known as the Sinovac COVID-19 vaccine).
An emphasis on neutralization assays
In this study, volunteers employed in two health care institutions in Santiago (Chile) received two doses of CoronaVac – each dose being administered 28 days apart (in accordance with the Chilean vaccination program). Patient samples were acquired between May and June 2021.
Pseudotyped virus neutralization assays were performed in order to study functional antibody responses against SARS-CoV-2 in laboratory conditions. The researchers calculated the percentage of neutralization for each dilution, as well as the infectious dose ID50, which is an estimated number of viral particles necessary to produce infection in 50% of individuals.
This study also pursued public data analysis by using available data on SARS-CoV-2 lineages from the Consorcio Genomas CoV2 site in Chile, and vaccination data published by the Chilean Ministry of Science, Technology, Knowledge and Innovation.
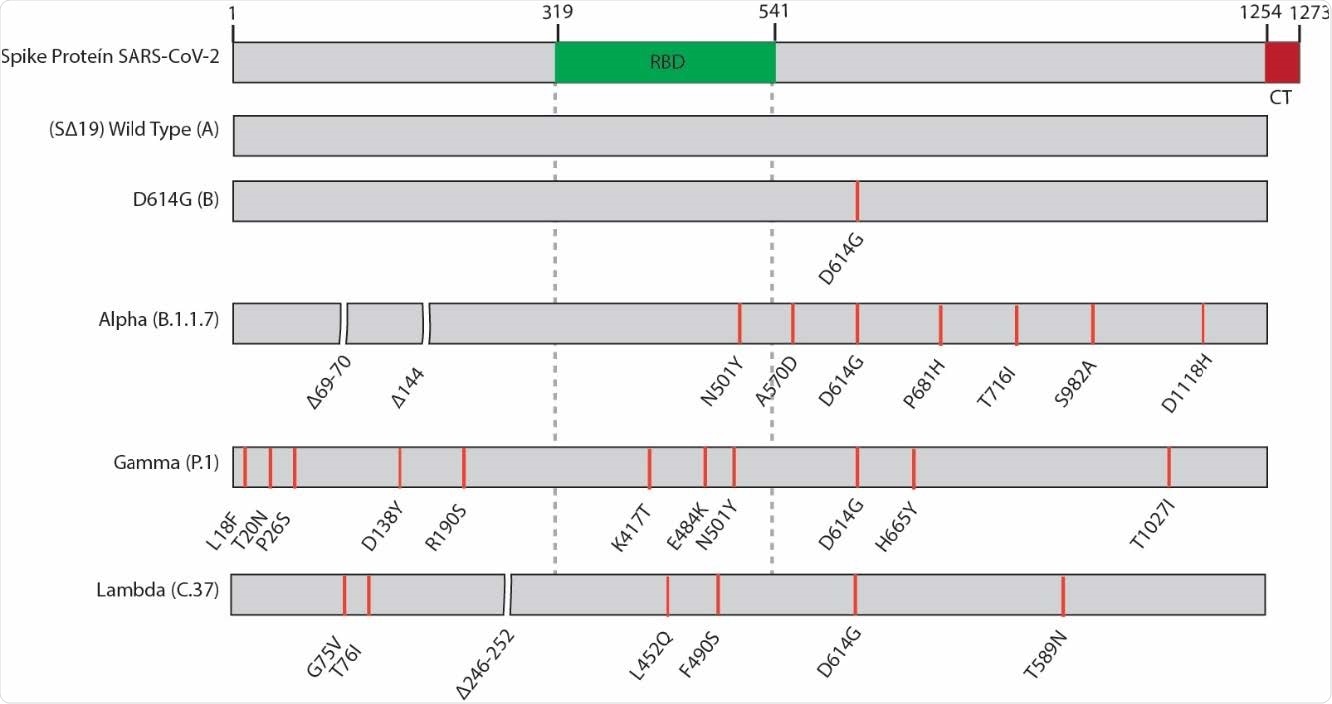
Schematic representation of the SARS-CoV-2 spike protein and the variants used in this study. Lineages are indicated in parenthesis. RBD, receptor-binding domain, CM; cytoplasmic tail.
Increased infectivity and escape from neutralization
The researchers observed an increased infectivity potential of the variant of interest mediated by the Lambda spike glycoprotein that was even higher in comparison to the D614G (lineage B) or the Alpha and Gamma variants.
More specifically, when compared to the wild-type virus (i.e., Wuhan-1 reference lineage A), the neutralization potential was diminished by 3.05-fold for the Lambda variant. For comparison purposes, this was 1.37-fold lower for the D614G, 2.03-fold lower for the Alpha variant and 2.33-fold lower for the Gamma variant.
No correlation between age, sex, smoke status, or body mass index and neutralizing antibody titers has been observed in this study cohort, which means that this should be considered a relatively universal phenomenon.
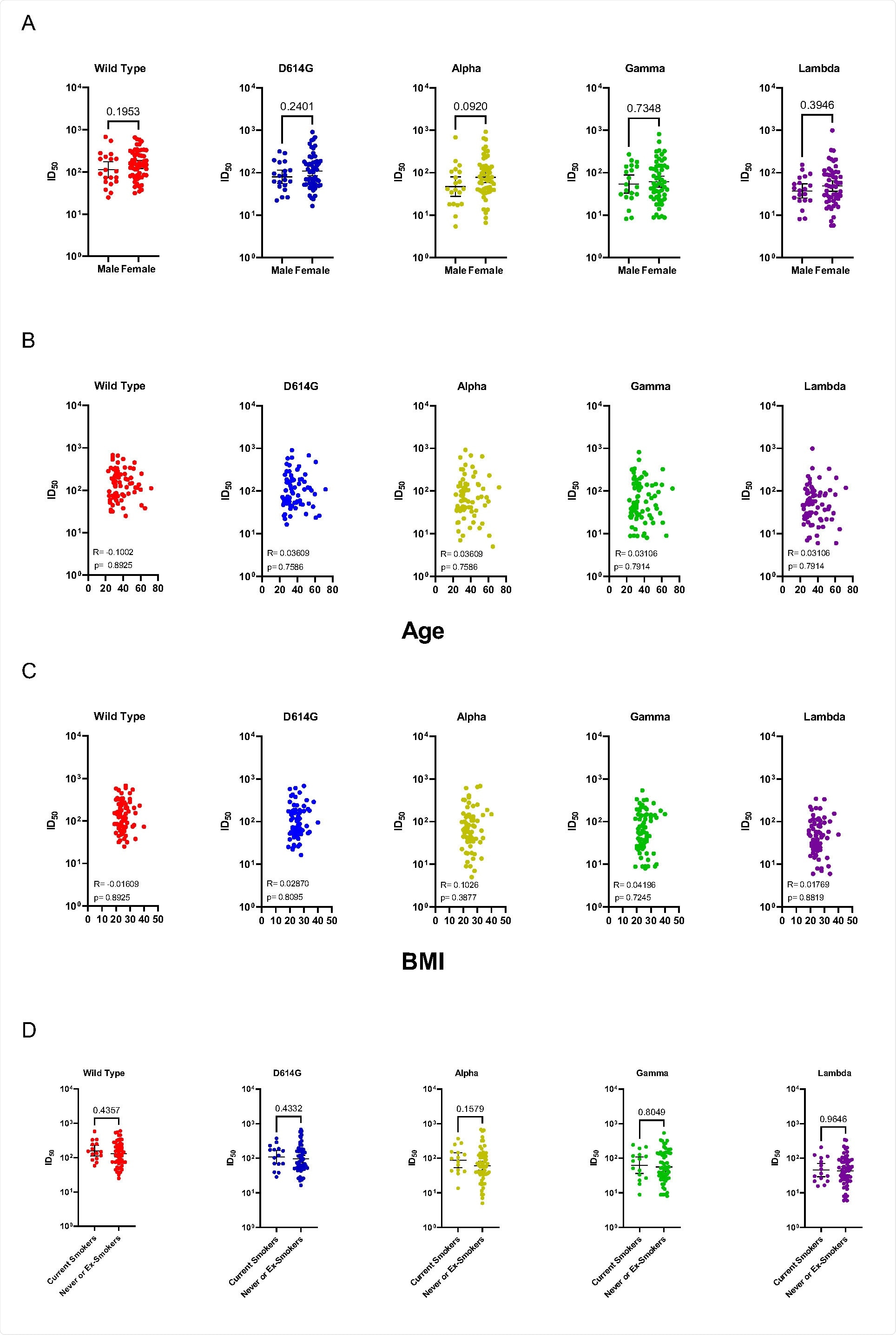
Correlation of neutralizing antibody titers against SARS-CoV-2 variants and participants’ sex, age, BMI or smoke status. Stratification neutralizing antibody titers (NAbTs) against SARS-CoV-2 variants by sex (A), Age (B),
The need for strict genomic surveillance
In a nutshell, these results indicate that mutations present in the spike glycoprotein of the Lambda variant of interest give rise to increased infectivity and enable the immune escape of this specific lineage from neutralizing antibodies elicited by CoronaVac.
“These data reinforce the idea that massive vaccination campaigns in countries with high SARS-CoV-2 circulation must be accompanied by strict genomic surveillance allowing the identification of new isolates carrying spike mutations and immunology studies aimed to determine the impact of these mutations in immune escape and vaccines breakthrough”, caution study authors in this medRxiv paper.
Such propensity of variants to escape from vaccine-induced immunity means there is still a need for next-generation vaccines that would provide broader neutralizing activity against present and potential future SARS-CoV-2 variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Acevedo, M.L. et al. (2021). Infectivity and immune escape of the new SARS-CoV-2 variant of interest Lambda. medRxiv. https://doi.org/10.1101/2021.06.28.21259673, https://www.medrxiv.org/content/10.1101/2021.06.28.21259673v1.full
- Peer reviewed and published scientific report.
Acevedo, Mónica L., Aracelly Gaete-Argel, Luis Alonso-Palomares, Marco Montes de Oca, Andrés Bustamante, Aldo Gaggero, Fabio Paredes, et al. 2022. “Differential Neutralizing Antibody Responses Elicited by CoronaVac and BNT162b2 against SARS-CoV-2 Lambda in Chile.” Nature Microbiology 7 (4): 524–29. https://doi.org/10.1038/s41564-022-01092-1. https://www.nature.com/articles/s41564-022-01092-1.