Researchers in Germany have provided evidence that a booster shot of a messenger RNA (mRNA)-based coronavirus disease 2019 (COVID-19) vaccine following prime immunization with AstraZeneca’s adenoviral vector-based vaccine is sufficient to achieve high levels of neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The team – from the University Hospital Erlangen, the University Hospital of Cologne, and the Technical University of Munich – says that this heterologous prime-boost regimen using different COVID-19 vaccines may even increase the efficacy of vaccination, while providing an alternative approach to immunization in settings where vaccines are in short supply.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Concerns surrounding two doses of AstraZeneca vaccine
Administration of the first dose of AstraZeneca’s adenoviral vector-based ChAdOx1 nCoV-19 vaccine is associated with a risk for developing vaccine-induced immune thrombotic thrombocytopenia (VITT), also called thrombosis thrombocytopenia syndrome (TTS). This complication is estimated to occur in between 1 and 2 cases per 100,000 vaccinations, with younger women having been reported as the highest risk group.
This risk of VITT has also been reported for Janssen’s Ad26.COV2.S product – another adenoviral-vector-based COVID-19 vaccine.
“As vaccine-induced antibodies against platelet factor 4 have been implicated in the pathogenesis of VITT, an amplifying effect of a second vaccine dose cannot be excluded,” writes Ulrike Protzer and colleagues.
Several countries have therefore suggested that the second AstraZeneca dose should be replaced by an mRNA-based vaccine as a precautionary measure, although few or even no data regarding the safety and efficacy of this heterologous prime-boost regimen are available.
What did the researchers do?
Given this lack of data, Protzer and colleagues performed anti-SARS-CoV-2 antibody testing for 229 individuals who had received a heterologous vaccination using ChAdOx1 nCoV-19 as the prime immunization and Pfizer-BioNTech’s mRNA-based BNT162b2 vaccine as the booster shot.
The levels of neutralizing antibodies that were achieved in this group were compared with those among cohorts of healthcare workers or volunteers, who were immunized using a homologous BNT162b2 or homologous ChAdOx1 nCoV-19 regimen, respectively.
The team performed a culture-based SARS-CoV-2 neutralization assay using Vero E6 cells. They also conducted a surrogate neutralization assay that detects competition for binding to the SARS-CoV-2 spike protein receptor-binding domain (RBD) between serum antibodies and recombinant angiotensin-converting enzyme 2 (ACE2). The viral spike protein mediates the initial stage of the infection process when its RBD attaches to the host cell receptor ACE2. This spike RBD is the primary target of neutralizing antibodies following SARS-CoV-2 vaccination or natural infection.
What did they find?
The team observed a striking increase in the levels of neutralizing antibodies against SARS-CoV-2 among the 229 vaccinees who received a booster dose of BNT162b2 between 9 and 12 weeks after receiving a prime dose of ChAdOx1 nCoV-19.
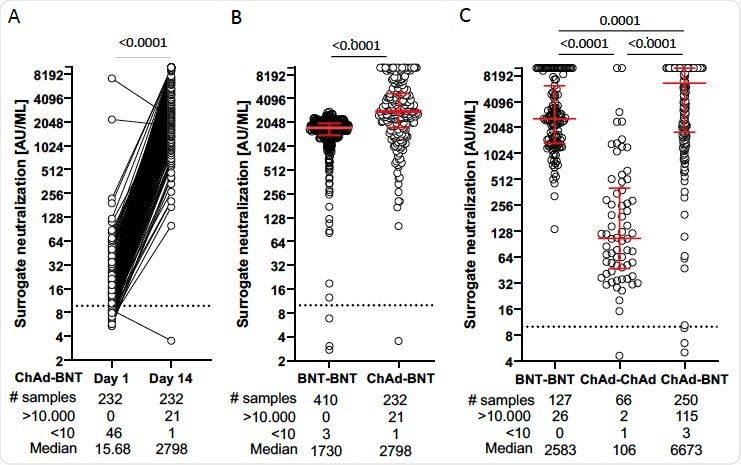
Comparison of surrogate neutralization activity induced by homologous and heterologous COVID-19 vaccine regimens. Vaccinees received either BNT162b2 mRNA (BNT) or ChAdOx1 nCoV-19 (ChAd) vaccine. (Panel A) In vaccinees who received ChAd as a prime and BNT as a boost vaccine, serum samples were obtained at the day of boost vaccination and at day 14±1 after the heterologous booster. Surrogate neutralization activity is given in paired samples. (Panel B) Dot plots showing surrogate neutralization activity from a group of vaccinees receiving homologous vaccination with BNT (BNT - BNT) compared to that receiving heterologous vaccination (ChAd – BNT) determined by laboratory 1. (Panel C) Dot plots of surrogate neutralization activity after homologous BNT - BNT, ChAd - ChAd and heterologous ChAd - BNT vaccination determined by laboratory 2. Dots represent single vaccinees. P values from a two-tailed Wilcoxon matched-pairs signed-rank test (A), a two-tailed Mann Whitney test (B), and a Dunns multiple comparisons test (C) are shown above the graph. Median and interquartile ranges in (B) and (C) are indicated by red horizontal lines. Descriptive statistics shown below the graph include numbers (#) of samples, median and number of samples with results below the lower (<10) and above the upper (>10.000) cut-off of the surrogate neutralization assay.
Serum samples were analyzed on the day of boosting with BNT162b2 and two weeks thereafter.
Analysis of all 480 individuals at the two-week time point revealed that the heterologous prime-booster regimen induced significantly higher neutralizing antibody titers than the homologous ChAdOx1 nCoV-19 or homologous BNT162b2 regimens.
The heterologous regimen may increase vaccine efficacy
The team says the study findings show that administering a single dose of a COVID-19 mRNA vaccine as a booster following prime vaccination with ChAdOx1 nCoV-19 is sufficient to achieve high levels of neutralizing antibodies against SARS-CoV-2.
“These results obtained in over 480 individuals, who received a prime with an adenoviral vector and a boost with an mRNA vaccine, indicate that a heterologous prime-boost with different COVID-19 vaccines may increase vaccine efficacy, and offers an alternative in a setting of vaccine shortage,” says Protzer and colleagues.
“Further studies, however, need to address the safety and clinical efficacy of heterologous vaccination regimens,” they add.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Protzer U, et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2 mRNA. medRxiv, 2021. doi: https://doi.org/10.1101/2021.07.03.21258887, https://www.medrxiv.org/content/10.1101/2021.07.03.21258887v1
- Peer reviewed and published scientific report.
Tenbusch, Matthias, Sofie Schumacher, Emanuel Vogel, Alina Priller, Jürgen Held, Philipp Steininger, Stephanie Beileke, et al. 2021. “Heterologous Prime–Boost Vaccination with ChAdOx1 NCoV-19 and BNT162b2.” The Lancet Infectious Diseases 21 (9): 1212–13. https://doi.org/10.1016/s1473-3099(21)00420-5. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00420-5/fulltext.