A recent study by a team of scientists at Beth Israel Deaconess Medical Center and Janssen Vaccines & Prevention has demonstrated that humoral and cellular immunity elicited by the coronavirus disease 2019 (COVID-19) vaccine Ad26.COV2.S remains active for at least eight months.
Moreover, the vaccine exhibits potent neutralizing efficacy against more infectious variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including B.1.1.7 (Alpha), B.1.351 (Beta), B.1.617.2 (Delta), and P.1 (Gamma) variants. The study is currently available on the medRxiv* preprint server while awaiting peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The Ad26.COV2.S is a recombinant, replication-incompetent, human adenovirus type 26 (Ad26) vector-based COVID-19 vaccine that contains prefusion-stabilized, full-length spike protein of SARS-CoV-2 as an immunogen.
In preclinical trials, the vaccine has been found to provide durable protection against SARS-CoV-2 infection even at low doses. Similarly, the observations made in early clinical trials have revealed that a single vaccine dose is safe to administer and capable of inducing a robust humoral and cellular immune response.
Regarding storage and transport advantages, Ad26.COV2.S can be stored for up to 2 years in standard freezers and up to 3 months at 4°C.
In the current study, the scientists evaluated the efficacy of Ad26.COV2.S vaccine in inducing humoral and cellular immune responses 8 months and 6 months after administering 1st and 2nd vaccine doses, respectively. Moreover, they investigated whether vaccine-induced antibodies are capable of neutralizing the original SARS-CoV-2 strain and its variants, including the B.1.1.7 (alpha), B.1.351 (beta), B.1.617.2 (delta), B.1.429 (epsilon), P.1 (gamma) and B.1.617.1 (kappa) variants.
Study design
A total of 20 individuals participated in the trial. Of them, 10 received a single vaccine dose (prime dose), and 10 received two vaccine doses (prime and booster doses). In addition, five participants received a placebo dose.
Humoral immune responses
In all vaccinated participants, the antibody responses were measured at days 29, 57, 71, and 239. A gradual increase in spike receptor-binding domain (RBD) specific antibody titer was observed at three early time points, followed by a slight reduction at day 239. Similarly, comparable neutralizing antibody titers were observed at all studied time points. Specifically, only a 1.8-fold reduction in neutralizing antibody titer was observed between day 71 (peak response) and day 239 (durability timepoint). Importantly, no significant difference in antibody titers was observed between participants who received a single vaccine dose or two vaccine doses.
Regarding the B.1.351 variants, more than a 13-fold reduction in neutralizing antibody titer was observed on day 29. However, at day 239, only a 3-fold reduction in antibody titer was observed compared to that for the original SARS-CoV-2 strain.
In participants who received only a single vaccine dose, the neutralizing antibody titers of 184, 158, 147, 171, 107, 129, 87, and 62 were observed against wildtype SARS-CoV-2 and variants D614G, B.1.1.7, B.1.617.1, B.1.617.2, P.1, B.1.429, and B.1.351, respectively, at day 239. In general, an induction in neutralizing antibody titer against SARS-CoV-2 variants was observed at day 239 compared to day 29.
Taken together, these observations indicate that the vaccine is capable of inducing a broad range of neutralizing antibodies against a number of SARS-CoV-2 variants over time.
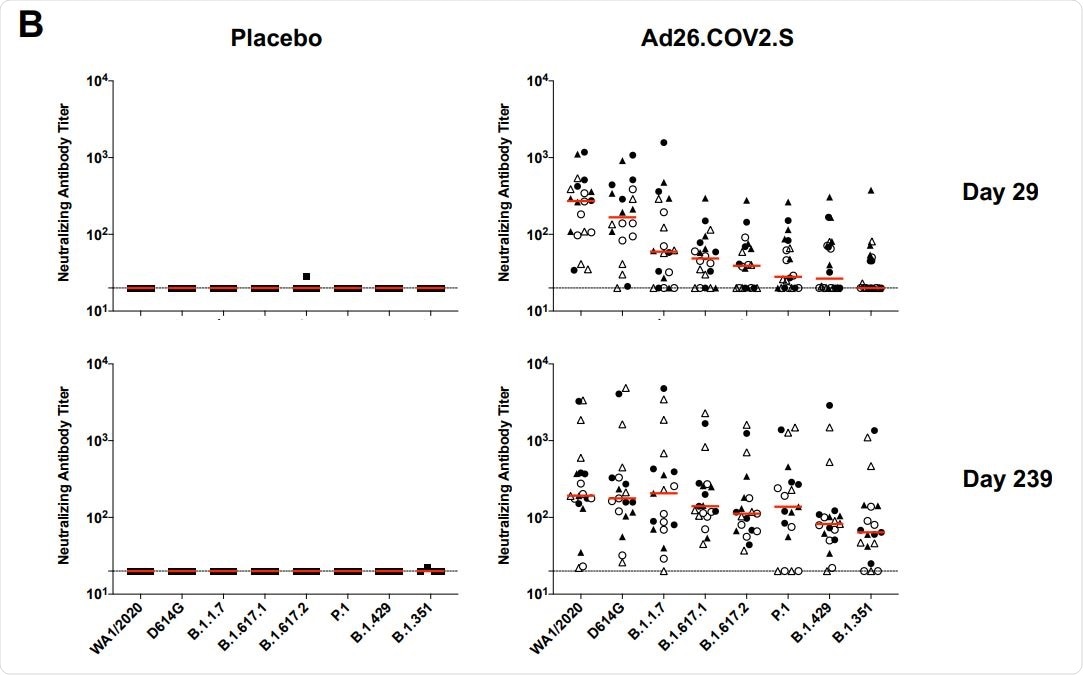
Durability of humoral and cellular immune responses following Ad26.COV2.S vaccination. Pseudovirus neutralizing antibody assays against the parental WA1/2020 strain as well as the SARS-CoV-2 variants D614G, B.1.1.7 (alpha), B.1.617.1 (kappa), B.1.617.2 (delta), P.1 (gamma), B.1.429 (epsilon), and B.1.351 (beta) on days 29 and 239.
Cellular immune responses
Intracellular cytokine staining assays were conducted to measure spike-specific, IFNγ-secreting CD4+ and CD8+ T cell responses in vaccinated participants at days 57, 85, and 239.
The findings revealed a gradual induction of CD8+ T cell responses that did not decline over 8 months in vaccinated participants. However, a slight reduction in CD4+ T cell response was observed between day 57 and day 239.
Study significance
The study reveals that the Ad26.COV2.S COVID-19 vaccine induces robust and durable humoral and cellular responses with a negligible decline for at least eight months following immunization. Importantly, a gradual induction in neutralizing antibody level and range has been observed in participants who received a single vaccine dose. This indicates maturation of B cell responses even without a booster dose.
Given the high efficacy against a wide range of viral variants, Ad26.COV2.S is expected to be a potential future vaccine against COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Barouch DH. 2021. Durable Humoral and Cellular Immune Responses Following Ad26.COV2.S Vaccination for COVID-19. medRxiv, https://doi.org/10.1101/2021.07.05.21259918, https://www.medrxiv.org/content/10.1101/2021.07.05.21259918v1
- Peer reviewed and published scientific report.
Barouch, Dan H., Kathryn E. Stephenson, Jerald Sadoff, Jingyou Yu, Aiquan Chang, Makda Gebre, Katherine McMahan, et al. 2021. “Durable Humoral and Cellular Immune Responses 8 Months after Ad26.COV2.S Vaccination.” The New England Journal of Medicine, July. https://doi.org/10.1056/NEJMc2108829. https://www.nejm.org/doi/10.1056/NEJMc2108829.