Researchers in the United States have designed a modular protein subunit vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that elicited a strong immune response in macaques and prevented the animals from developing coronavirus disease 2019 (COVID-19).
The spike RBD mediates the initial stage of the SARS-CoV-2 infection process when it binds to the host cell receptor angiotensin-converting enzyme 2. This RBD is a primary target of neutralizing antibodies following natural infection or vaccination.
Dan Barouch from Harvard Medical School in Boston, Massachusetts and colleagues say the RBD-VLP antigen elicited significantly higher levels of neutralizing antibodies than other licensed vaccines have in non-human primates.
The vaccine also reduced viral loads in the upper and lower respiratory tract of the animals following challenge with SARS-CoV-2.
Furthermore, both the spike RBD and hepatitis B (VLPs) protein components are produced in yeast, potentially providing a low-cost manufacturing process for low- and middle-income countries, where access to vaccination is limited.
“Based on these promising data, this vaccine candidate is currently being tested in clinical trials,” says Barouch and colleagues.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Access to vaccines is limited in low-and middle-income countries
The mass rollout of COVID-19 vaccination in many developed countries has proved highly effective at reducing SARS-CoV-2 infection rates and preventing symptomatic COVID-19.
However, the availability of vaccines in low- and middle-income countries is limited, owing to insufficient supplies, high costs, and cold storage requirements.
New vaccines that could be produced by existing local manufacturers could minimize the costly infrastructure required for vaccine distribution to help achieve global immunity against SARS-CoV-2.
Protein subunit vaccines represent a promising solution, since they can be manufactured using local large-scale fermentation facilities, do not typically require cold storage, and are already known to safe and effective when used with adjuvants.
What did the team do?
Now, Barouch and colleagues have described the design and preclinical testing of the protein subunit vaccine – RBD-VLP – that is currently manufactured at the Serum Institute of India production facility.
“Both protein components are produced in yeast, making this vaccine a promising, low-cost intervention for low- and middle-income countries,” says the team.
The researchers used a polypeptide-based system called SpyTag-SpyCatcher to covalently link the antigen to the VLP, which has previously been shown to increase antigen-specific antibody titers in mice.
“The modularity of the SpyTag-SpyCatcher system allows each component of the final particle to be expressed and purified independently to maximize yields and quality,” they explain.
The RBD-VLP antigen was formulated with one of two adjuvants: aluminum hydroxide (alum) and a combination of alum with CpG1018 – a potent commercial adjuvant that is known to elicit T helper 1 (Th1)-type cytokine responses.
Cynomolgus macaques were immunized with two doses of either vaccine formulation or a placebo, separated by a three-week interval. Spike-specific antibody titers were measured following each dose.
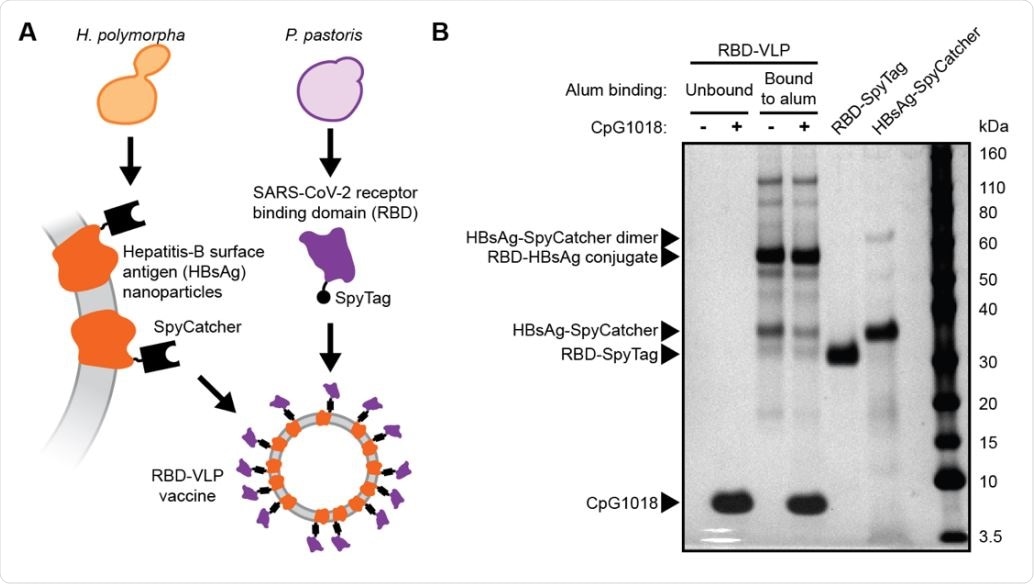
Design and analysis of the RBD-VLP drug product A) Schematic of protein expression and conjugation. B) Reduced SDS-PAGE analysis of the formulated RBD-VLP vaccine samples. Alum-bound protein antigen (with and without CpG) was separated by centrifugation and desorbed from the alum using an elution buffer combined with heat treatment prior to SDS-PAGE.
What did the study find?
The researchers say they observed titers of neutralizing antibodies among the immunized animals that were more than 10,000 times above the range of protection provided by other licensed vaccines previously tested in non-human primates.
The alum-only formulation elicited significantly higher antibody levels than the combination adjuvant. However, the alum plus CpG1018 combination improved the cellular immune response, compared with alum alone.
“The cellular response appeared stronger with the alum and CpG1018 co-formulation, consistent with previous reports on the influence of CpG1018 as an adjuvant in vaccines,” says the team.
The researchers say that it has previously been demonstrated that CpG adjuvants with hepatitis B surface antigen vaccines boost the Th1 cellular response and complement the Th2 cellular response that is induced by alum alone.
“In agreement with these prior reports, we did observe a higher Th1 cell response to the RBD-VLP formulated with both alum and CpG1018 compared to alum only,” they write.
The vaccine reduced viral loads in the respiratory tract
The researchers infected the macaques with SARS-CoV-2 two weeks following the second dose of vaccine or placebo.
Among the immunized animals, there was an approximate 3.4 and 2.9 log10 reduction in the median viral of bronchoalveolar lavage supernatants and nasal samples, respectively, compared with the control animals.
Animals that received the sham vaccine showed evidence of interstitial inflammation, syncytial cells, and type II pneumocyte hyperplasia in the lung and a higher median cumulative pathology score, compared with the immunized animals.
“Notably, post-challenge levels of humoral immunity conferred by the vaccine were not significantly different between the two vaccine formulations,” says the team.
What did the authors conclude?
Barouch and colleagues say that taken together, the results suggest that the RBD-VLP vaccine candidate is protective against SARS-CoV-2 infection.
“Based on these promising data, this vaccine candidate is currently being tested in clinical trials (ANZCTR Registration number ACTRN12620000817943),” they write.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Barouch D, et al. A modular protein subunit vaccine candidate produced in yeast confers protection against SARS-CoV-2 in non-human primates. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.07.13.452251, https://www.biorxiv.org/content/10.1101/2021.07.13.452251v1
- Peer reviewed and published scientific report.
Dalvie, Neil C., Lisa H. Tostanoski, Sergio A. Rodriguez-Aponte, Kawaljit Kaur, Sakshi Bajoria, Ozan S. Kumru, Amanda J. Martinot, et al. 2022. “SARS-CoV-2 Receptor Binding Domain Displayed on HBsAg Virus–like Particles Elicits Protective Immunity in Macaques.” Science Advances 8 (11). https://doi.org/10.1126/sciadv.abl6015. https://www.science.org/doi/10.1126/sciadv.abl6015.