Researchers in the United States have demonstrated early and persistent B cell abnormalities in patients with moderate or severe coronavirus disease 2019 (COVID-19) that may be largely driven by hypoxia.
Given that the B cell response is a crucial part of the host immune defense against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the causative agent of COVID-19 – these deficits could have a significant impact on the disease course, says Kenneth Smith from the University of Cambridge and colleagues.
“Hypoxia might contribute to B cell pathology in COVID-19 and in other hypoxic states,” writes the team. “Through this mechanism, it may impact on COVID-19 outcome, and be remediable through early oxygen therapy.”
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Hypoxia and lymphopenia prominent clinical features in COVID-19
Hypoxia is a prominent but “silent” early feature of COVID-19, with patients often presenting at hospital with low blood oxygen saturation and respiratory failure.
Another early prominent clinical feature in cases of moderate-to-severe COVID-19 is pronounced lymphopenia, with significant reductions in B cell subsets.
“This brought to mind the phenotype of mice with von Hippel-Lindau (VHL)-deficient B cells, in which Hypoxia-Inducible Factors (HIFs) are constitutively active, suggesting hypoxia might drive B cell abnormalities in COVID-19,” write Smith and colleagues.
The B cell response is a vital component of the immune reaction to SARS-CoV-2, with B cell-induced antibodies contributing to protection from infection.
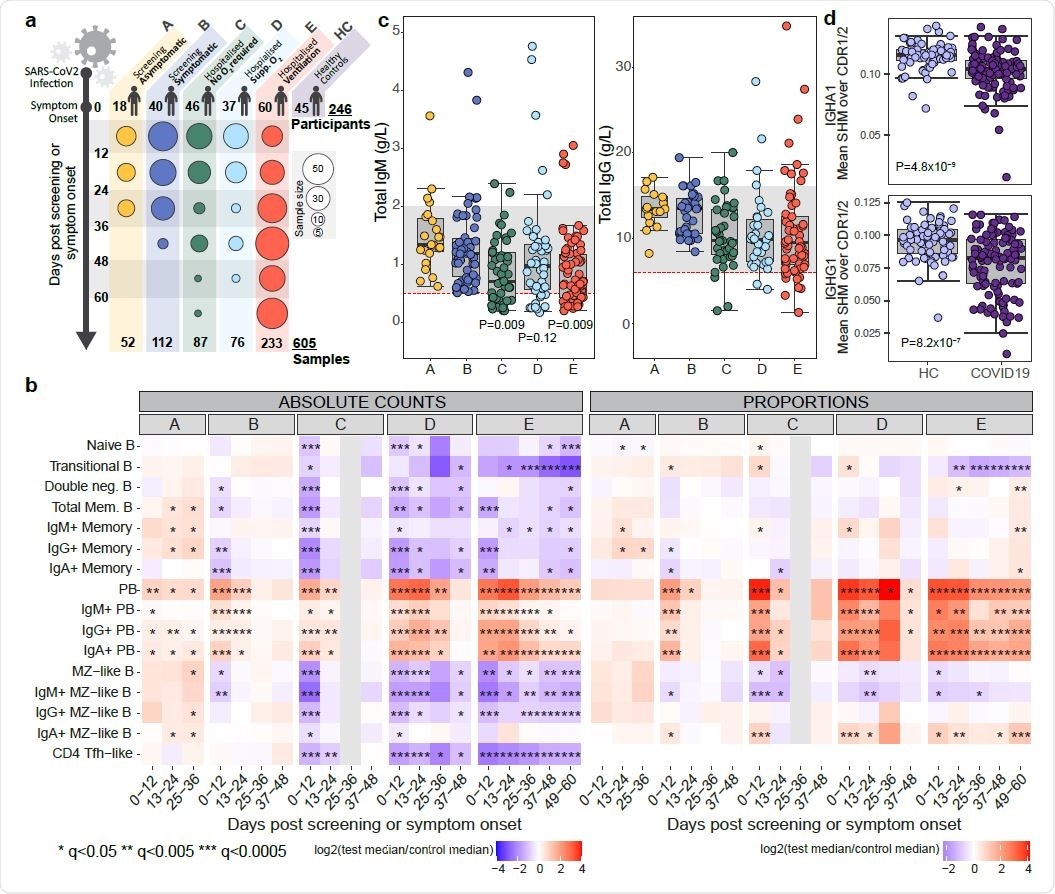
The prominent changes in B cell subsets that occur in symptomatic COVID-19 include an increase in plasmablasts and a reduction in memory B cells that correlate with disease severity and may persist for more than two months following symptom onset.
B cell abnormalities in COVID-19 may limit immune response to SARS-CoV-2
The B cell abnormalities that occur in moderate-to-severe COVID-19 may limit the efficacy of the immune response to infection with SARS-CoV-2 and predispose to reinfection or to secondary infection with other pathogens, the latter being a significant clinical problem in COVID-19.
“Understanding the changes to B cell immunity caused by COVID-19, and the potential role of hypoxia as a mechanism underlying them, could therefore inform clinical management strategies,” say the researchers.
Cells respond to hypoxia via the HIF isoforms: HIF-1α and HIF-2α. In the presence of oxygen, these transcription factors are tagged for degradation by prolyl-hydroxylase enzymes (PHDs) and Von Hippel Lindau (VHL) E3 ubiquitin ligase. In the hypoxic state, reduced PHD activity leads to stabilization of HIF-α.
“B cell adaptation to hypoxia may be physiologically important. Germinal centers (GCs) are hypoxic, and mice with B cell-specific VHL deletion and thus constitutively active HIF show abnormal B cell development and reduced GC B cells, antibody class-switching and affinity-maturation,” says Smith and colleagues.
What did the researchers do?
The team explored the potential impact of hypoxia on B cells in cohorts of SARS-CoV-2-infected individuals recruited between March 31st and July 20th, 2020.
The participants were categorized by peak clinical severity into asymptomatic individuals, those with mild symptoms, patients who presented to hospital but never required oxygen supplementation, hospital admissions who required oxygen, and admissions who required assisted ventilation or died without ventilation.
Blood samples were taken from the participants on a weekly basis while they were inpatients and on a less frequent basis following discharge. The researchers compared absolute B cell subset numbers between COVID-19 patients and healthy controls.
What did they find?
Among asymptomatic individuals, an often non-significant increase in most B cell subsets was observed. Among those with mild symptoms, short-lived reductions in immunoglobulin A (IgA) and IgG memory B cells and in marginal zone-like (MZL) B cells were observed. In both of these groups, early increases in plasmablasts were seen, which then slowly declined.
By contrast, significant early reductions in many B cell subsets were observed in all more severe disease groups, including reductions in naïve and transitional B cells, memory B cells and MZL B cells.
Most B cells subsets then exhibited some recovery, although transitional B cells continued to fall in cases of severe disease.
B cells in COVID-‐19
When the researchers performed a comparative analysis in mice with VHL-deficient B cells, they found a remarkably similar B cell dysregulation to that observed in patients with moderate-to-severe disease.
The researchers say this finding supports the possibility that signaling via HIF can contribute to B cell abnormalities that resemble those seen in COVID-19.
Next, the team found evidence of a hypoxia transcriptional signature that occurs in moderate-to-severe COVID-19, which single-cell analysis revealed to be particularly enriched in B cells.
Furthermore, when the researchers explored B cell immune response in mice housed in hypoxic conditions, they found that hypoxia contributed to the B cell defects that are also seen in COVID-19, including reduced marginal zone and germinal center B cells.
What did the authors conclude?
The team says the study shows that profound B cell abnormalities occur in patients with severe COVID-19 and provides evidence that the deficits may be largely driven by hypoxia.
“B cell lymphopenia extends across all subsets, is present soon after symptom onset, and is often persistent,” writes Smith and colleagues.
The researchers also say that the observation that hypoxia perturbs B cell immunity has implications in a wide range of clinical settings.
“In COVID-19, these observations lead to the prediction that early and aggressive oxygen therapy may lead to improved immune responsiveness and thus clinical outcome,” they conclude.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Smith K, et al. The impact of hypoxia on B cells in COVID-19. medRxiv, 2021. doi: https://doi.org/10.1101/2021.07.12.21260360, https://www.medrxiv.org/content/10.1101/2021.07.12.21260360v1
- Peer reviewed and published scientific report.
Kotagiri, Prasanti, Federica Mescia, Aimee L. Hanson, Lorinda Turner, Laura Bergamaschi, Ana Peñalver, Nathan Richoz, et al. 2022. “The Impact of Hypoxia on B Cells in COVID-19.” EBioMedicine 77 (March). https://doi.org/10.1016/j.ebiom.2022.103878. https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(22)00062-7/fulltext.