Following the burden and spread of SARS-CoV-2 infection during the coronavirus disease (COVID-19) pandemic is of utmost importance in small environments, but also larger geographical settings. Basically, all of our current data is derived following the use of two different classes of assays.
Active disease is diagnosed with the methods that detect the presence of virus in upper respiratory specimens – most notably viral nucleic acid amplification tests (with real-time polymerase chain reaction being the gold standard) and immunodetection of viral antigen.
Conversely, we can also detect the response of our immune systems by detecting virus-specific antibodies, which is done by serological or antibody-based assays. These are precisely the methods that should be used in seroepidemiology, as they can demonstrate prior infections as well.
Antibody-based assays for SARS-CoV-2 are based on two viral antigens: spike glycoprotein that attaches to the host cell receptor (angiotensin-converting enzyme 2 or ACE2) by utilizing RBD in the S1 subunit, and nucleocapsid, which interacts with viral RNA inside the envelope.
Thus far, assays that used nucleocapsid antigen had a preference in a majority of clinical settings, while spike glycoprotein has been chiefly used as antibody capture antigen in research milieus. But is it time for more pervasive clinical applications of spike glycoprotein-based serological assays?
In this new paper, a research group led by Dr. Pratik Datta delineates key traits of their serological enzyme-linked immunosorbent assay (ELISA), which utilizes a novel S1 RBD antigen, as well as its propensity to detect antibodies in minimal amounts of remotely collected peripheral blood.
A comparative study approach
The assays described in this study were performed using a novel gp70-fusion protein form of the S1 RBD antigen. The gp70 domain is characteristic for its chaperone-like qualities, which means it aids in the assembly or disassembly of other macromolecular structures.
Consequently, the researchers compared detection properties of virus-specific antibody responses to SARS-CoV-2 infection of their RBD-based ELISA assay with two specific assays from Roche Elecsys and Abbott Architect that utilize nucleocapsid antigen as the capture reagent.
Furthermore, since different study designs can result in specimen collection of either serum or plasma from peripheral blood, this research group has also conducted a matrix equivalency test to see whether the assay is amenable to different types of liquid specimens (including non-blood bodily fluids).
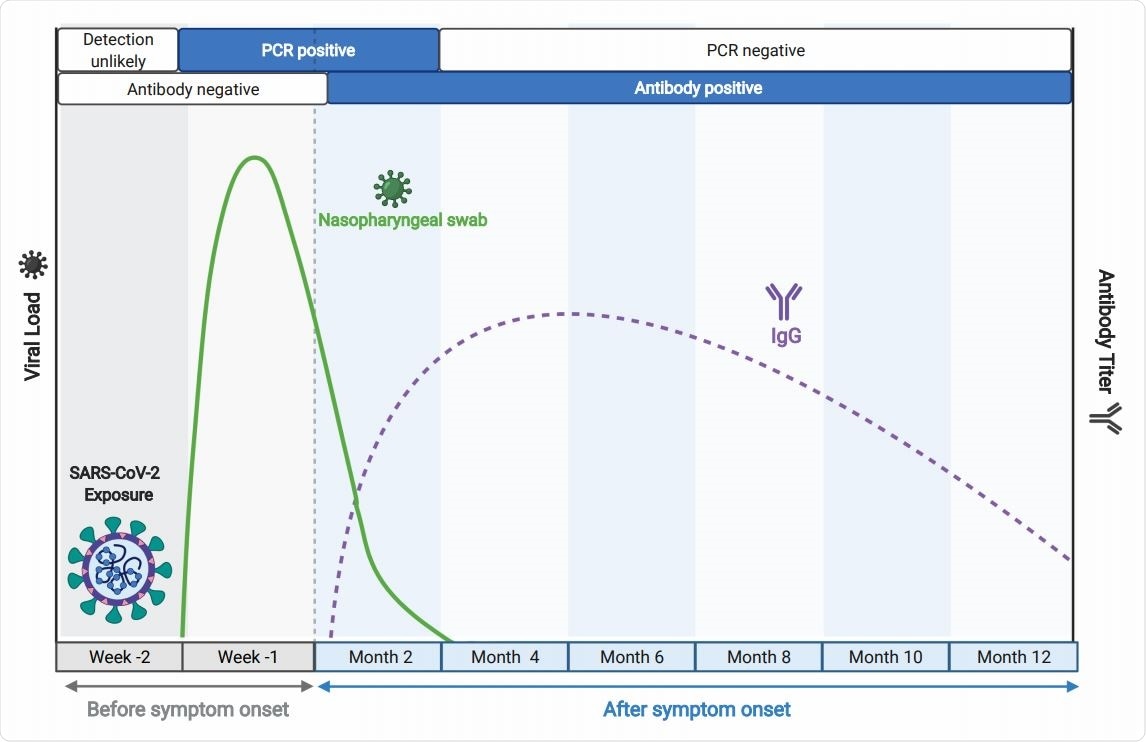
Time course of key biomarkers in SARS-CoV-2 infection, adapted from BioRender.com. The solid green line represents a typical trajectory of the RT-PCR data for viral nucleic acid from respiratory samples, while the broken purple line indicates a typical virus-specific antibody trajectory in peripheral blood, relative to time of infection, as indicated.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Characteristics of a versatile assay
In short, albeit the sample size was somewhat limited in this study, the results have shown that this RBD-based ELISA assay has excellent sensitivity and specificity, as well as a favorable profile in comparison compares with commercial tests that are currently widespread in clinical settings.
Moreover, the researchers have shown that microsampling and phlebotomy can be used interchangeably for peripheral blood collection. In contrast, the utilization of serum or plasma from different blood collection tubes had no demonstrable effect on antibody-binding results.
The results also imply that this ELISA protocol can be compatible with various matrices, including serum, plasma acquired using different anticoagulants, and non-blood bodily fluids such as breast milk. All of this makes it very adaptable to a diverse array of study designs.
Improving seroepidemiology research
Overall, the paper neatly demonstrates how this type of ELISA test based on spike glycoprotein RBD can be accurate, adaptable, and highly fitting for a myriad of research and clinical applications.
“Our protocol is performed utilizing robotic sample handling and dilution and automated ELISA,” further explain the authors of this bioRxiv manuscript. “Moreover, it compares favorably with accurate commercial tests that have been widely used in clinical practice to determine exposure to SARS-CoV-2”, they add.
All of this means that its use is remarkably suited for seroepidemiology approaches and other large-scale studies that necessitate parsimonious sample collection outside of healthcare settings. Considering the further spread of SARS-CoV-2, practical and versatile solutions are a welcome addition to our armamentarium.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Datta, Pratik, Rahul Ukey, Natalie Bruiners, William Honnen, Mary O. Carayannopoulos, Charles Reichman, Alok Choudhary, et al. 2021. “Highly Versatile Antibody Binding Assay for the Detection of SARS-CoV-2 Infection and Vaccination.” Journal of Immunological Methods 499 (December): 113165. https://doi.org/10.1016/j.jim.2021.113165. https://www.sciencedirect.com/science/article/pii/S0022175921002106?via%3Dihub.
- Peer reviewed and published scientific report.
Datta, Pratik, Rahul Ukey, Natalie Bruiners, William Honnen, Mary O. Carayannopoulos, Charles Reichman, Alok Choudhary, et al. 2021. “Highly Versatile Antibody Binding Assay for the Detection of SARS-CoV-2 Infection and Vaccination.” Journal of Immunological Methods 499 (December): 113165. https://doi.org/10.1016/j.jim.2021.113165. https://www.sciencedirect.com/science/article/pii/S0022175921002106?via%3Dihub.