Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the etiological agent of COVID-19 (coronavirus disease 2019). To date, the COVID-19 pandemic has resulted in over 190 million confirmed SARS-CoV-2 infections and over 4 million COVID-related deaths.
The SARS-CoV-2 infection is mainly contracted from airborne virions. The primary site of infection in the human body is the nasopharyngeal epithelium, particularly the ciliated cells. The epithelial lining of the nose is an essential portal for infections from viruses. It is also an important site for viral replications.
Therefore, the nose is an important target for prophylaxis and also for therapy. In a recent study posted to the bioRxiv* preprint server, Fabio Fais et al. developed a safe and easy-to-use nasal spray, AM-301, a medical device marketed as Bentrio, to protect against infection by SARS-CoV-2 and potentially other viruses.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The researchers tested the safety and efficacy of AM-301 against SARS-CoV-2 and showed that it is safe on the nasal epithelium. It also significantly reduced the viral titer in this study.
The mechanism of action of AM-301 is physical, i.e., the components are neither metabolized nor absorbed and thus non-pharmaceutical. As an added benefit, the researchers noted that the spray might be used against a wide range of viruses, allergens, and pollutants in the air.
Using a natural substance with broad pharmaceutical applications and virus-capturing properties such as the clay, the researchers developed a simple and safe intervention to protect and enhance the nasal barrier function against SARS-CoV-2.
Bentonite
Bentonite is a clay mineral composed of thin aluminum silicate sheets with a net negative charge. The team developed a nasal spray containing bentonite (magnesium aluminum silicate) in a matrix composed of mono-, di- and triglycerides, propylene glycol, xanthan gum, mannitol, disodium EDTA, citric acid and water.
Even though bentonite has been known for many years to be capable of capturing viruses, this study represents its first application in capturing airborne viruses.
The formulation AM-301 is characterized as a white to light beige, odorless and tasteless, aqueous gel emulsion with a pH of 6.0. “When we applied it to our own nasal mucosa or palmar skin, it had a soothing non-irritant, lotion-like consistency,” explained the researchers in the paper.
“Seeking to minimize potential side effects and facilitate frequent and compliant use, we selected only inert ingredients such as pharmaceutical excipients and substances that are generally recognized as safe.”
MucilAir
Using a well-established model of primary human nasal epithelium, MucilAir, the researchers demonstrated that the AM-301 could: 1) prevent MucilAir from being infected by SARS-CoV-2, and 2) mitigate an established infection in MucilAir without any previous treatment.
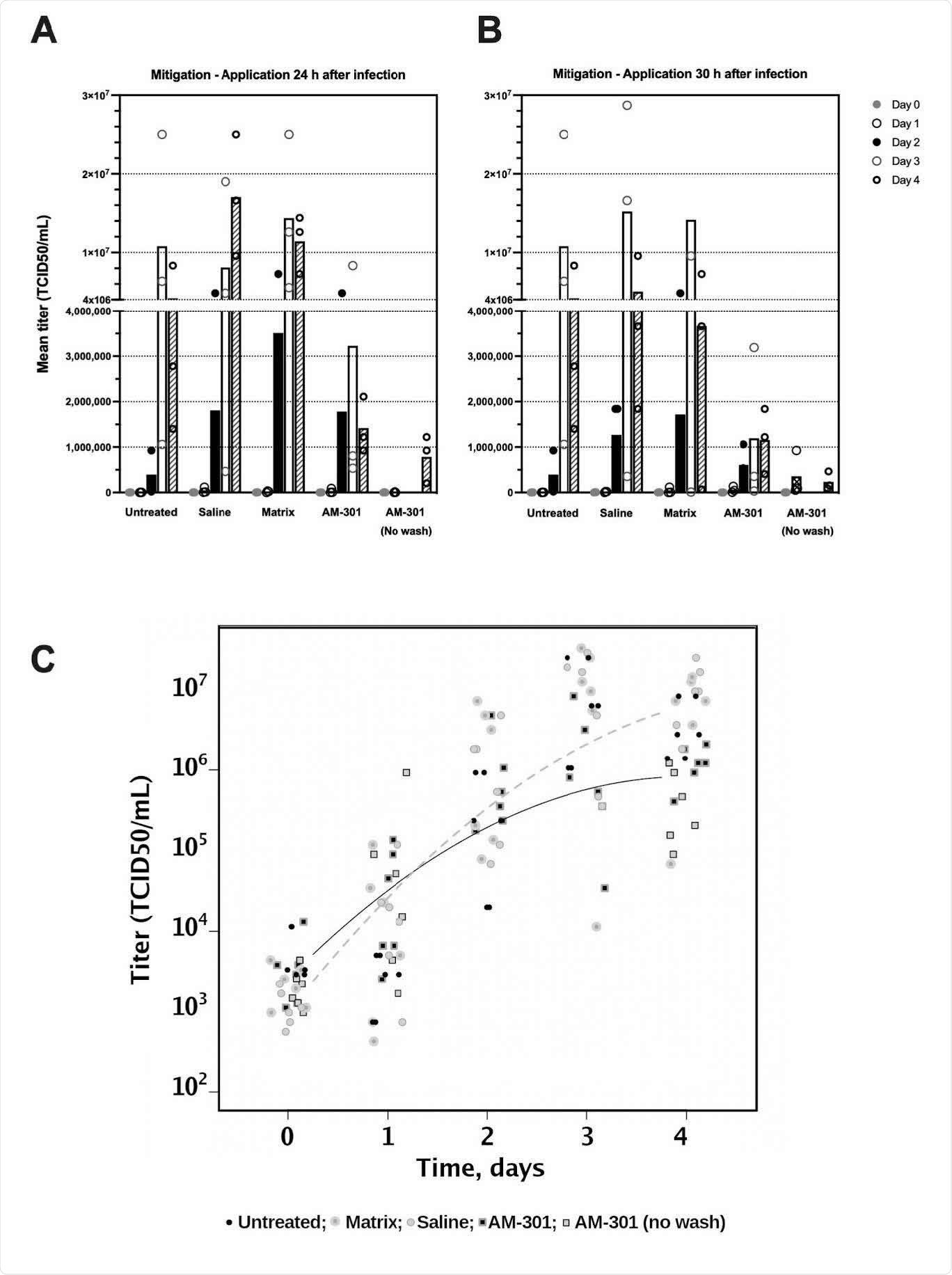
AM-301 in the mitigation of SARS-CoV-2 infection. A, B Bar charts with mean values and individual data points. Test substances were applied 24 or 30 h after the start of the experiment (Protocols 1 and 2, respectively). C Linear mixed-effect model. The log-linear scatter plot shows individual log transformed data and concave curves for negative control samples (untreated, saline- and matrix-treated inserts; dashed curve) and for AM-301-treated inserts (continuous curve) (t=3.68, p<0.01). The model shows a deceleration in exponential growth, as is typically observed for sigmoidal behaviors.
Despite repeated applications for 4 days, AM-301 did not cause any detrimental effect on MucilAir, except a slight reduction in cilia beating frequency. The researchers attributed this may be due to the viscosity of the preparation.
“The positive effects of AM-301 in reducing SARS-CoV-2 infection are supported by the observations from linear-mixed effect models, which showed significant decelerations in viral titer growth in both the prophylaxis and mitigation assays.”
In the initial stages of SARS-CoV-2 infection, it is known that the nasal tracts are infected with a high concentration of the virus. As a result, the infected person is highly contagious and at risk of developing, by aspiration along the naso-oropharynx-lung axis, a lower respiratory infection. “The nasal microenvironment, therefore, plays a major role in SARS-CoV-2 infection and clinical course,” observed the researchers.
In the nasal tract, two lines of protection are provided - a physical one, epithelium of the nasal mucosa, and immune protection provided by immune cells resident in the nasopharynx-associated lymphoid tissue.
“Together, mucociliary clearance and immune responses should protect the nasal epithelium from pathogens, but infection can ensue in cases of high viral exposure or dysfunction of these mucosal defenses.”
Despite the robust nasal barrier, viruses often succeed in invading the body. Presently, a therapeutic solution is unavailable. AM-301, as a non-pharmacological nasal spray may be used to prevent SARS-CoV-2 infection or mitigate existing infection.
The encouraging results of this study call for further investigations in vivo and in humans to further evaluate AM-301 as a medical device with a broad spectrum of action against a battery of viruses, allergens, and pollutants, the researchers write.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Drug-free nasal spray as a barrier against SARS-CoV-2 infection: safety and efficacy in human nasal airway epithelia, Fabio Fais, Reda Juskeviciene, Veronica Francardo, Stéphanie Mateos, Samuel Constant, Massimo Borelli, Ilja P. Hohenfeld, Thomas Meyer, bioRxiv 2021.07.12.452021; doi: https://doi.org/10.1101/2021.07.12.452021, https://www.biorxiv.org/content/10.1101/2021.07.12.452021v1
- Peer reviewed and published scientific report.
Fais, Fabio, Reda Juskeviciene, Veronica Francardo, Stéphanie Mateos, Manuela Guyard, Cécile Viollet, Samuel Constant, Massimo Borelli, and Ilja P. Hohenfeld. 2022. “Drug-Free Nasal Spray as a Barrier against SARS-CoV-2 and Its Delta Variant: In Vitro Study of Safety and Efficacy in Human Nasal Airway Epithelia.” International Journal of Molecular Sciences 23 (7): 4062. https://doi.org/10.3390/ijms23074062. https://www.mdpi.com/1422-0067/23/7/4062.