Researchers in Canada have reported the discovery of a multi-antibody cocktail that synergistically protected against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in an animal model of acute infection.
The SARS-CoV-2 virus is the agent responsible for the coronavirus disease 2019 (COVID-19) pandemic that continues to threaten global public health and the worldwide economy.
In vitro experiments showed that the antibody cocktail targeted diverse non-overlapping epitopes on the SARS-CoV-2 spike protein – the main structure the virus uses to bind to and infect host cells.
When administered to hamsters, it suppressed replication-competent viral titers to undetectable levels in the animals’ lungs.
While administering two of the individual cocktail components as monotherapies also showed clear in vivo protection, neither component achieved the efficacy observed with the multi-antibody TATX-03.
“To our knowledge, we are the first to report on the discovery of a multi-antibody cocktail, beyond a dual therapy, that produces in vivo protection against SARS-CoV-2 via synergistic effects in an animal model of infection,” says the team from ImmunoPrecise Antibodies Ltd., in Victoria, British Columbia.
Furthermore, the researchers showed that TATX-03 maintained in vitro neutralizing activity against the B.1.1.7 (alpha), B.1.351 (beta) and P.1 (gamma) variants of concern.
“These results merit further development of TATX-03 as a potential therapy for SARS-CoV-2 infection with resistance to mutagenic escape,” writes Ilse Roodink and colleagues.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
Current antibody therapies are susceptible to mutagenic escape
Since the SARS-CoV-2 outbreak first began in late December 2019, the virus has infected more than 192 million people and caused more than 4.13 million deaths.
While mass vaccination is being rolled out in many countries, more effective and sustainable therapeutic strategies are needed to prevent and treat COVID-19.
To date, four antibody therapeutics that target the SARS-CoV-2 spike protein have received emergency use authorization, including two monoclonal antibodies and two dual-antibody therapies.
“However, the exquisite specificity of these antibodies also renders them susceptible to mutagenic escape,” says Roodink and colleagues.
Most therapeutic monoclonal antibody candidates target the receptor-binding domain (RBD) of the spike protein and disrupt its interaction with the human angiotensin-converting enzyme 2 (ACE2) receptor to neutralize viral entry.
However, the epitopes of these antibodies are mostly clustered at or near the ACE2-binding interface of the RBD, where mutations could evolve that confer resistance to this neutralization.
Using any of these antibodies as monotherapies significantly increases this risk says the team.
“However, a lack of epitope diversity in the dual-antibody cocktails developed by Regeneron (casirivimab and imdevimab) and Eli Lilly (bamlanivimab and etesevimab) leaves both antibodies in each cocktail potentially susceptible to evasion by single point mutations.”
What about multi-antibody cocktails?
A combination of three or more monoclonal antibodies that target diversified non-overlapping epitopes has previously been shown to provide prophylactic and therapeutic protection in other infectious diseases, including Ebola, respiratory syncytial virus, rabies, and botulinum.
“In addition to minimizing their risk of mutagenic escape, multi-antibody cocktails can stimulate complementary mechanisms of action in concert, unlocking synergistic effects that potentially allow for a lower effective clinical dose than in a monotherapy approach,” says Roodink and colleagues.
What did the researchers do?
The team used pre-existing human B cell libraries to identify a diverse panel of SARS-CoV-2 spike-specific antibodies that reacted to a broad range of spike epitopes, thereby providing versatile options for a combination therapy.
The researchers prioritized TATX-03 – a combination of antibodies that target distinct, non-overlapping spike epitopes.
The multi-antibody cocktail was constructed as two alternate 4-antibody formulations for the assessment of efficacy in vitro.
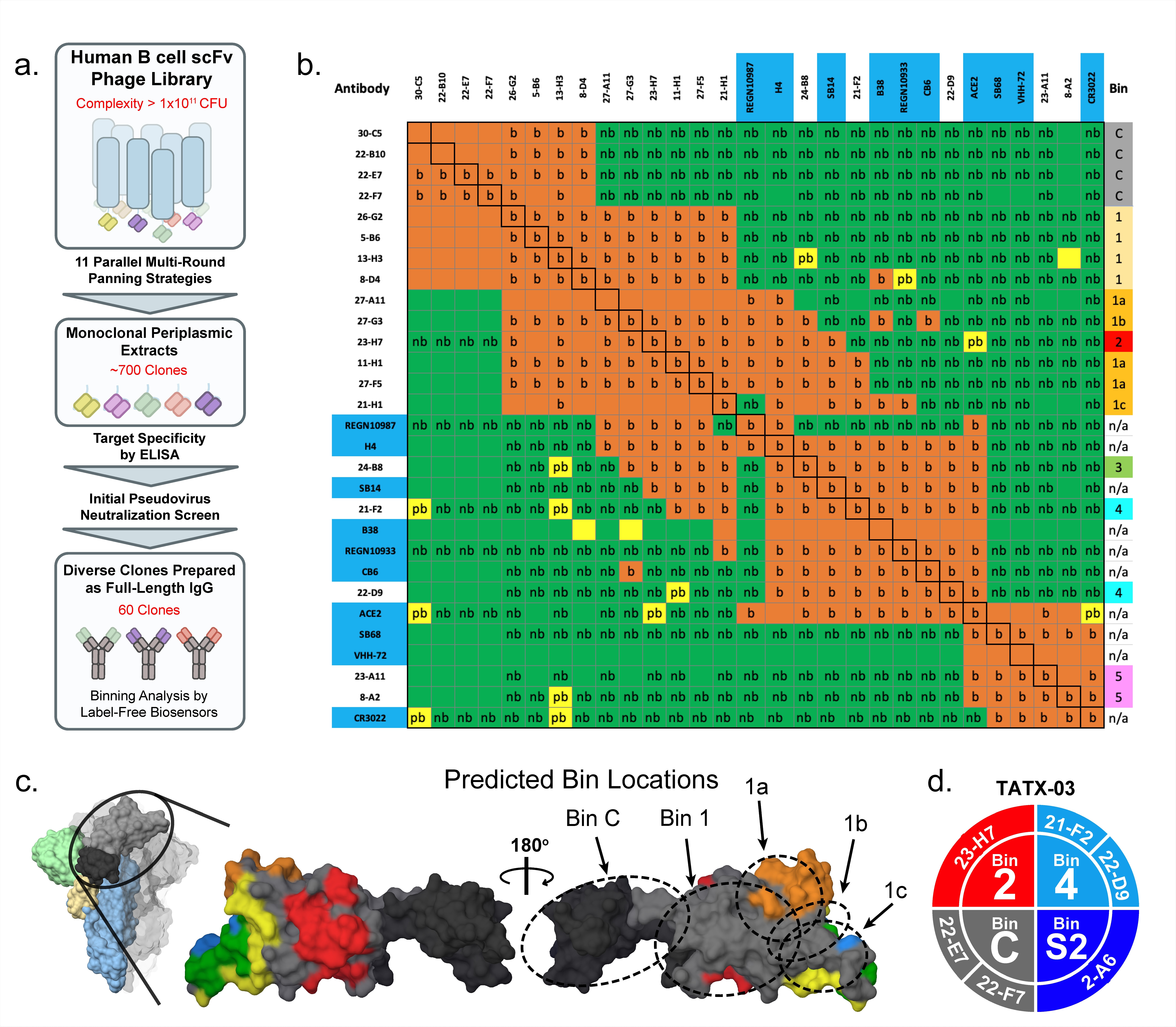
IPA’s library-to-leads triage process. (a) High-level schematic of the workflow. (b) Heat map for a pairwise analysis of 19 library-derived anti-SARS-CoV-2 S1-specific Abs merged with a panel of ten structural benchmarks (9 literature Abs and ACE2). The red, yellow, and green colored cells indicate Ab pairs that are blocked (b), partially blocked (pb), or not blocked (nb), respectively. Colored cells with a designation of “b, pb, or nb” were measured empirically, whereas those without a designation are “inferred”. The black boxed cells along the diagonal indicate the “self blocked” pairs. In our bin-definition, bin-members block one another and show similar blocking behaviors when tested against other Abs in the panel. RBD-specific clones were assigned to five bins (1-5). RBD binders that did not block ACE2 were assigned bin “1”, which was split into sub bins (bin 1a, 1b, and 1c) based on their nuanced blockade towards the structural benchmarks

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The cocktails synergistically neutralized SARS-CoV-2
Using authentic virus-based neutralization assays, both formulations synergistically neutralized SARS-CoV-2; only one antibody component (in TATX-03b) exhibited neutralizing effects independently.
Next, the team determined the efficacy of the cocktails and their individual components in a Syrian hamster model of acute SARS-CoV-2 infection, which also revealed that both formulations provided protection.
The TATX-03 cocktail reduced titers of replication-competent viral titers to undetectable levels in the lungs of the animals following both prophylactic and therapeutic administration.
Monotherapy with two of the individual antibody components also exhibited in vivo protection. However, neither antibody was able to recapitulate the efficacy of TATX-03.
This synergistic effect was further supported when the in vivo efficacy of these individual antibodies and corresponding cocktails were examined at a lower dose.
Furthermore, in vitro screening of vesicular stomatitis virus (VSV) particles pseudotyped with spike proteins representing the SARS-CoV-2 lineages B.1.1.7, B.1.315, and P.1, revealed that TATX-03 maintained potent neutralizing activity against these variants of concern.
The team calls for further development of TATX-03
“We are the first to describe a multi-antibody cocktail, beyond dual therapy, that shows synergistic effects both in vitro and in vivo and confers protection in an in vivo animal model of SARS-CoV-2 infection in both prophylactic and therapeutic settings,” writes the team.
Furthermore, “we show that TATX-03 is broadly resistant to emerging variants of concern in vitro and we anticipate that it retains in vivo efficacy towards these mutants, thereby meriting its further development as a potential therapy for the prevention or treatment of SARS-CoV-2 infection,” concludes Roodink and colleagues.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.