The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the causal agent of coronavirus disease 19 (COVID-19), emerged in December 2019 in Wuhan, China, and continues to spread worldwide. Dialysis patients (DP) and kidney transplant recipients (KTR) are especially vulnerable to COVID-19, as these patients experience a high percentage of complicated cases and a much higher mortality rate as compared to the normal population.
The messenger ribonucleic acid (mRNA) vaccines by Pfizer/BioNTech (BNT162b2) and mRNA-1273 (Moderna) have undergone extensive clinical trials and demonstrated a very high efficacy of about 95% after two doses. However, the clinical trials on these vaccines have mainly been conducted in the normal population, thereby leading to scarce vaccination data available for DP and KTR.
A new study published in The Lancet Regional Health - Europe analyzes the humoral and cellular immune responses of more than 3,100 Saxonian participants comprised of medical personnel (MP), as well as immunocompromised DP and immunosuppressed KTR patients. The humoral and cellular response rates and qualities were analyzed 4-5 weeks after the participants received their second vaccine dose of either vaccine.
About the study
To assess a pure vaccine-induced immune response, scientists constructed a “pure vaccination” cohort and a “clinical vaccination” cohort. The clinical vaccination cohort included all study participants, which consisted of pure vaccination cohort participants as well as individuals with COVID-19 disease (symptomatic or otherwise), strictly during or after vaccination.
The start date (T0) was defined as immediately before the first vaccination. Comparably, T1 was defined as three (Pfizer/BioNTech) to four (Moderna) weeks after the first dose, whereas T2 was defined as eight weeks after the start of the study or five to four weeks after the booster vaccine dose.
For all participants, SARS-CoV-2 specific immunoglobulin G (IgG)- or IgA-antibody reactions against the S1 and S2 subunits of the spike protein and the IgG- antibodies against the nucleocapsid protein subunit (NCP), at T0 and T2. Additionally, the receptor-binding domain (RBD) antibody formation was also examined at T2.
The measurements at T1 were taken for representative subgroups. De novo antibody development at T1 or T2 implied a positive serologic response. Scientists performed two independent assays including the SARS-CoV-2 specific interferon-γ release assay (IGRA) and in-depth immunophenotyping using flow cytometry (FACS) on the samples.
Study results
The pure vaccination cohort had 1,768 participants (144 MP, 1256 DP, and 368 KTR), all of whom were monitored at and up to eight weeks after vaccination. In the MP group, the seroconversion rate was 96% in T1 and 99% in T2. All participants in the MP group developed SARS-CoV-2 RBD antibodies at T2. Using the IGRA, scientists documented the cellular vaccination rate to be 81% at T1 and 86% at T2.
In the DP cohort, the seroconversion rate was also high at 95% at T2, with RBD antibodies detected in 95% of seroconversion positive DP cases at T2. Using IGRA, the cellular immune response rate was found to be 44% at T1 and 78% at T2. The researchers also observed a marked increase in the vaccine-reactive CD4+ T-cell response in the DP group at T2 as compared to pre-vaccination at T0 and T1.
Finally, for the KTR cohort, the seroconversion rate was quite low at about 8% at T1 and 42% at T2. As compared to the previous groups, the RBD antibody response rate in the KTR group was also low at 65%.
Post-vaccination, KTR patients showed a significant delay in T-cell responses, similar to DP patients. However, a marked increase was observed after the booster vaccine dose.
The KTR group showed much lower CD4+ T helper cells as compared to MP and DP. For the CD4+ T-cells producing interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), and interleukin 2 (IL-2), the kinetic patterns were similar in the DP and KTR cohorts in that there was a string delay from T0 to T2.
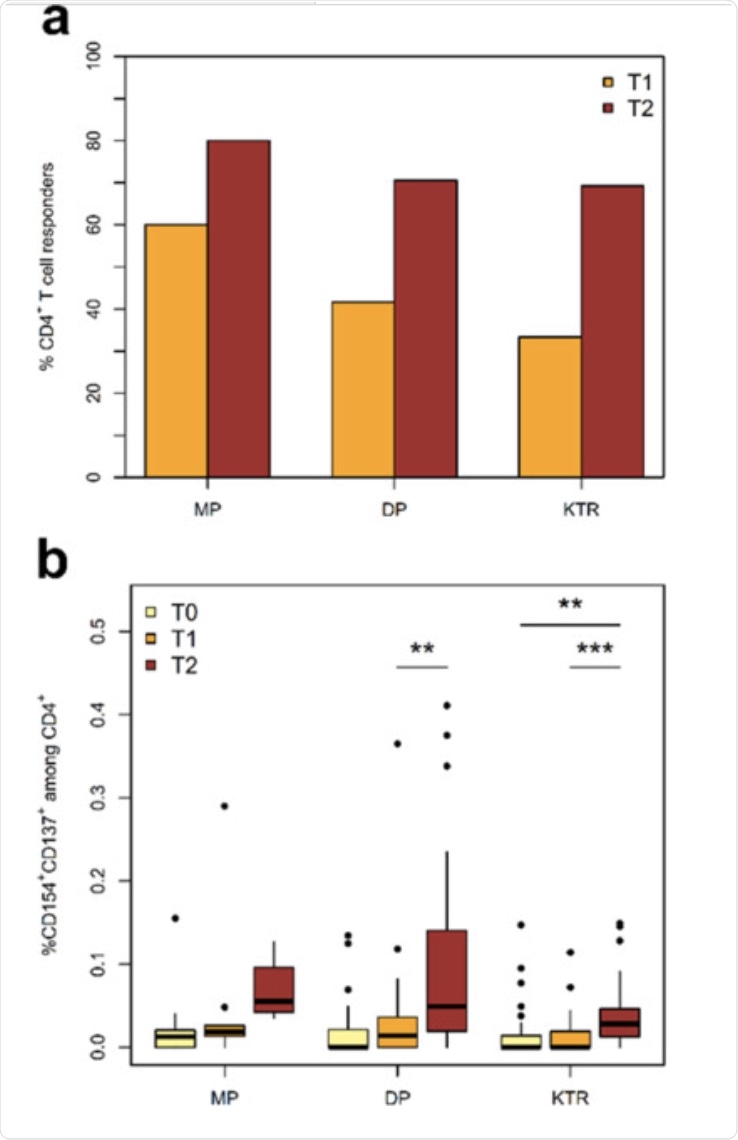 a) Incidence of SARS-CoV-2-reactive CD4+ T cell responders. A T-cell response is defined as a twofold increase or more in the frequency of activated T cells between T0 and T1 or T2. b) Kinetics of activated Spike-reactive CD4+ T helper cells at and following vaccination. Shown frequencies are after correction for background activation.
a) Incidence of SARS-CoV-2-reactive CD4+ T cell responders. A T-cell response is defined as a twofold increase or more in the frequency of activated T cells between T0 and T1 or T2. b) Kinetics of activated Spike-reactive CD4+ T helper cells at and following vaccination. Shown frequencies are after correction for background activation.
Conclusion
A thorough understanding of the vaccine-induced modifications in SARS-CoV-2 specific immunity can help scientists devise effective vaccination strategies to contain the ongoing pandemic.
In this study, scientists have shown that DP exhibit a very high seroconversion rate after boost vaccination. However, the humoral response is impaired in most of the patients who received transplants. The immunosuppressive drug number and the type of vaccine are important determinants of seroconversion failure, which suggests immune monitoring and altering of vaccination protocols.
Taken together, the results of the current study indicate that vaccination is safe but additional modifications to vaccination protocols, including booster doses for vulnerable individuals, should be considered.