A Korean study has recently demonstrated antiviral potency of monoclonal antibody CT-P59 against the delta variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The experiments conducted in animals infected with the delta variant have shown that CT-P59 is able to reduce symptom intensity and viral replication in the respiratory tract. The study is currently available on the bioRxiv* preprint server.
Novel SARS-CoV-2 variants belonging to the lineage B.1.617 have recently caused a significant induction in infection and mortality rates in India. Among three prominent sub-lineages of B.1.617, the delta variant (B.1.617.2) exhibits significantly higher infectivity and pathogenicity, and thus, is recognized as Variant of Concern (VOC) by the World Health Organization. Soon after its first identification in India, the delta variant has become dominant in many countries, including the USA and UK.
The mutations present in the spike receptor-binding domain (RBD) are mainly responsible for the high infectivity and immune evasion ability of novel SARS-CoV-2 variants. According to available literature, the L452R spike mutation of the delta and epsilon (first identified in California) variants is specifically associated with reduced viral neutralization by vaccine-induced antibodies and therapeutic monoclonal antibodies.
In the current study, the scientists have investigated the antiviral efficacy of the monoclonal antibody CT-P59 against the delta, Kappa (B.1.617.1), and epsilon (B.1.427/B.1.429) variants of SARS-CoV-2.
Mechanistically, the CT-P59 antibody binds to the viral spike protein and blocks its interaction with the host cell entry receptor angiotensin-converting enzyme 2 (ACE2). In both cell-based and animal studies, the antibody has shown significant antiviral efficacy against the original SARS-CoV-2 strain and the B.1.351 (first identified in South Africa) and P1 (first identified in Brazil) variants.
Neutralizing efficacy of CT-P59 in cell-based assays
The scientists determined binding affinity, antiviral ability, and neutralizing efficacy of CT-P59 against the delta, epsilon, and kappa variants with mutated RBDs. The findings revealed that the antibody has a 10-fold lower binding efficacy against the mutated RBDs when compared to that against the wild-type RBD.
Further experiments conducted using live or pseudotyped viruses revealed that the antibody has 183-fold, 74-fold, and 24-fold reduced antiviral ability against the delta, epsilon, and kappa variants, respectively, compared to that against the wild-type virus. Importantly, virus neutralization assay findings revealed that the antibody has 98-fold, 31-fold, and 50-fold reduced neutralizing efficacy against the delta, epsilon, and kappa variants, respectively, compared to that against the D614G variant of SARS-CoV-2.
Taken together, the findings reveal that despite relatively lower binding affinity and antiviral ability, CT-P59 is capable of neutralizing all tested variants of SARS-CoV-2.
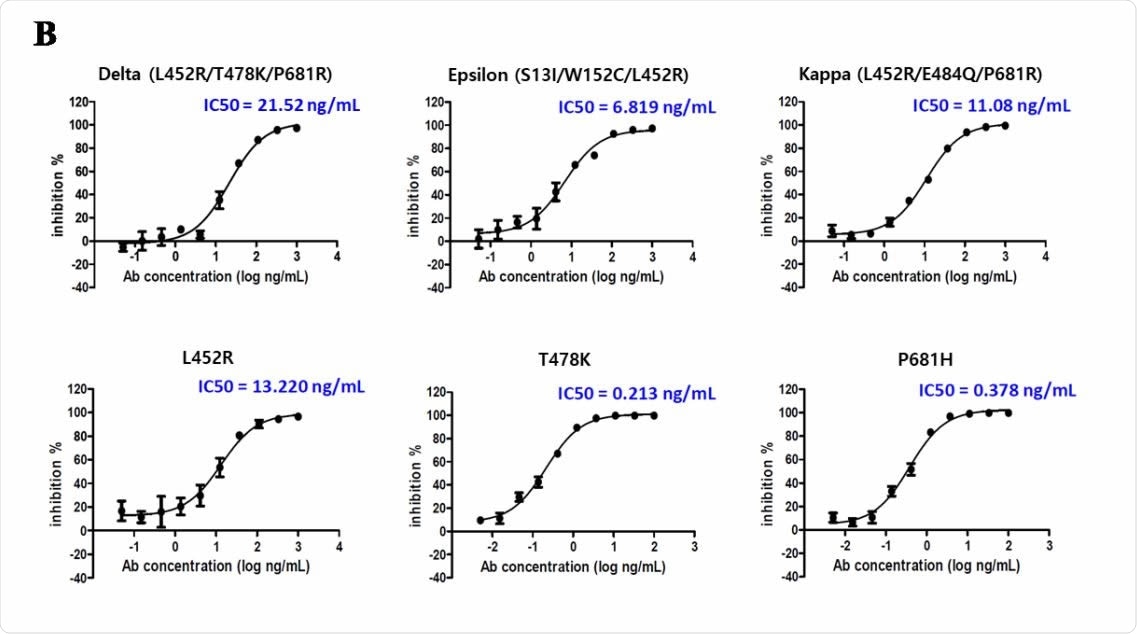
In vitro susceptibility test of CT-P59 against Delta, Epsilon, and Kappa variants.
Therapeutic efficacy of CT-P59 in animals
The scientists investigated the therapeutic efficacy of CT-P59 in mice infected with the delta variant. They used both high (clinically relevant) and low doses of the antibody to subsequently treat the infected mice.
The findings revealed that compared to untreated mice that showed a 0% survival rate at day 6 post-infection, all mice in the antibody-treated group showed a 100% survival rate. Moreover, mice treated with CT-P59 showed significantly less reduction in body weight compared to untreated control mice.
Importantly, CT-P59 treatment successfully reduced the viral replication in the lower respiratory tract of infected mice. Specifically, at day 6 post-infection, no viral titers were detected in the lungs of mice treated with both high and low antibody doses.
Study significance
The study findings reveal that despite exhibiting reduced antiviral efficacy via in vitro setups, the monoclonal antibody CT-P59 is capable of significantly ameliorating SARS-CoV-2-induced symptoms and reducing viral replication in the lungs of mice infected with the delta variant.
Based on the study findings, CT-P59 could be used in clinical setups to treat COVID-19 patients infected with the delta and associated variants with similar spike mutations.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
The in vitro and in vivo potency of CT-P59 against Delta and its associated variants of SARS-CoV-2 Dong-Kyun Ryu, Hye-Min Woo, Bobin Kang, Hanmi Noh, Jong-In Kim, Ji-Min Seo, Cheolmin Kim, Minsoo Kim, Jun-Won Kim, Nayoung Kim, Pyeonghwa Jeon, Hansaem Lee, Jeong-Sun Yang, Kyung-Chang Kim, Joo-Yeon Lee, Min-Ho Lee, Sang-Seok Oh, Hyo-Young Chung, Ki-Sung Kwon, Soo-Young Lee, bioRxiv, 2021.07.23.453472; doi: https://doi.org/10.1101/2021.07.23.453472, https://www.biorxiv.org/content/10.1101/2021.07.23.453472v1
- Peer reviewed and published scientific report.
Ryu, Dong-Kyun, Bobin Kang, Hanmi Noh, Sun-Je Woo, Min-Ho Lee, Patricia M. Nuijten, Jong-In Kim, et al. 2021. “The in Vitro and in Vivo Efficacy of CT-P59 against Gamma, Delta and Its Associated Variants of SARS-CoV-2.” Biochemical and Biophysical Research Communications 578 (November): 91–96. https://doi.org/10.1016/j.bbrc.2021.09.023. https://www.sciencedirect.com/science/article/pii/S0006291X21013176.