A population-based study conducted in Spain has revealed that both adenovirus vector-based and messenger ribonucleic acid (mRNA)-based coronavirus disease 2019 (COVID-19) vaccines are associated with an increased risk of venous thromboembolism (VTE) and thrombocytopenia (TCP). Moreover, the authors of this study highlight that the risk is relatively higher in men than women. The study is currently available on the medRxiv* preprint server.
Soon after deployment of the adenovirus-based COVID-19 vaccine developed by Oxford/AstraZeneca, a number of cases with thrombosis and TCP were reported among vaccine recipients from several countries. Based on the European Medicines Agency (EMA) report, the estimated risk of cerebral venous thrombosis associated with the AstraZeneca vaccine is 1 in 200,000 doses. Recently, the EMA has also identified some thromboembolic events in individuals who have received mRNA-based COVID-19 vaccines developed by Pfizer/BioNTech and Moderna.
Several recent studies have revealed that the risk of cerebral venous sinus thrombosis is 10-times higher in COVID-19 patients than in individuals immunized with mRNA vaccines. Surprisingly, some studies have claimed that mRNA vaccines (Pfizer and Moderna) are associated with a 10-fold higher risk of cerebral venous sinus thrombosis as compared to the AstraZeneca vaccine.
In the current study, the scientists have estimated the event rates of VTE, TCP, and VTE associated with TCP in partially or fully vaccinated individuals and COVID-19 patients. Specifically, the authors of this study have compared these event rates with corresponding reference rates in the same population using age and sex as covariates.
Study design
The study was conducted on three groups of individuals. In the first group, 662,719 individuals aged 10 years and above who received the 1st dose of AstraZeneca, Pfizer, or Moderna vaccines, were enrolled. Comparatively, the second group consisted of 622,778 individuals who received the 2nd dose of the Pfizer or Moderna vaccine.
In the third group, 190,616 patients with confirmed COVID-19 were included. As a reference group, a total of 7,013,040 individuals from the same population were analyzed.
The scientists estimated the event rates of various clinical forms of VTE and TCP, including cerebral venous sinus thrombosis, mesenteric thrombosis, portal vein thrombosis, or any VTE associated with TCP. They specifically focused on clinical events that occurred within 21 days following vaccination or COVID-19 diagnosis.
Important observations
The annual event rate of unusual site VTE, which includes cerebral venous sinus thrombosis, mesenteric thrombosis, and portal vein thrombosis, or VTE associated with TCP, in the reference population was estimated to be 2.15 per 100,000 individuals. The reference event rate of all tested clinical conditions increased with age and was higher in men than women. The event rates of unusual site VTE or VTE associated with TCP were estimated to be 5·65 and 7.23 per 100,000 doses in the first and second groups of this study, respectively.
Taken together, these observations revealed that the 1st dose of the COVID-19 vaccines was associated with an excess event rate of 2.5 cases per 100,000 for any VTE and VTE associated with TCP. Similarly, the 2nd dose of mRNA vaccines was associated with an excess event rate of 4 cases per 100,000 for any VTE and VTE associated with TCP.
In cases of mRNA vaccines, no significant difference in event rates was observed between the 1st and 2nd doses. However, the study could not differentiate between two doses of the AstraZeneca vaccine because of a lack of information.
In contrast to the vaccine groups, the event rate of unusual site VTE or VTE associated with TCP was estimated to be 32.53 per 100,000 patients in the COVID-19 group.
Regarding age and sex-related differences, the event rates of all tested clinical conditions were almost 2-fold higher in men than in women in both vaccine and COVID-19 groups. However, no significant correlation between age and event rate was observed among vaccinated individuals. In contrast, an increase in the event rate of VTE with age was observed among COVID-19 patients.
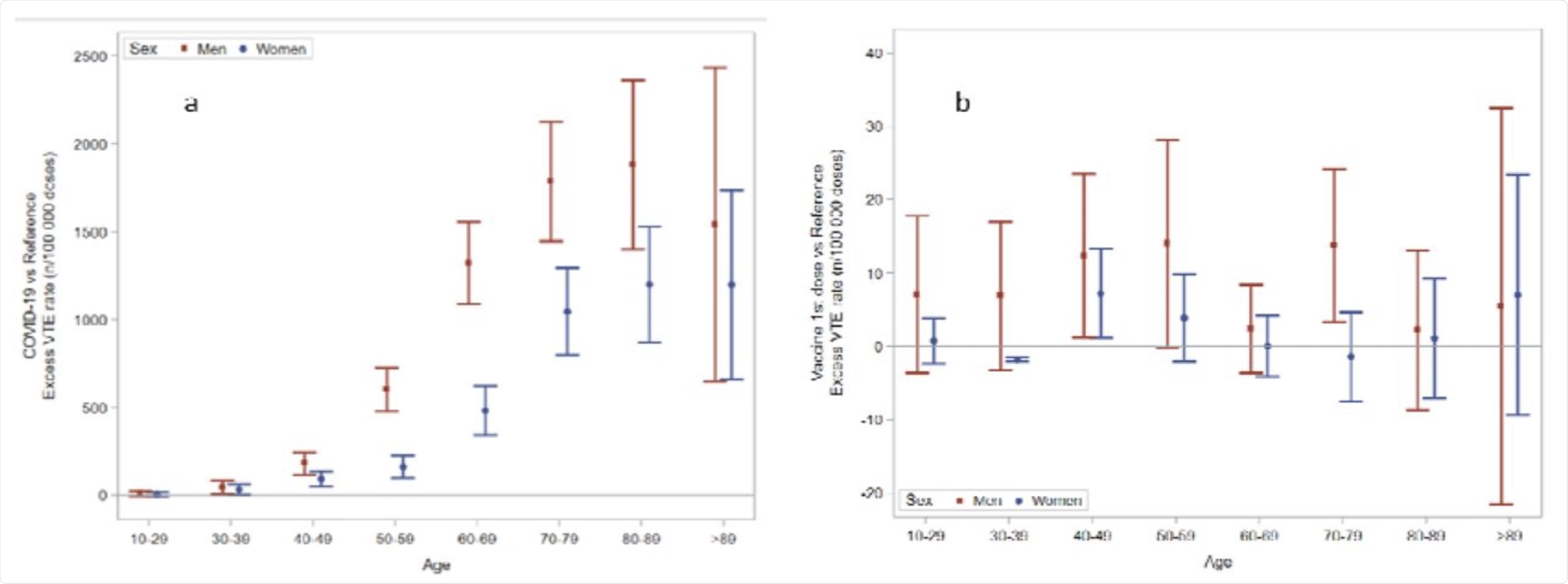 Excess rate of any venous thromboembolism by age and sex groups, in the Covid-19 cohort (a) and following the first dose of Covid-19 vaccines (b). The scale of the ordinal axis differs by 50-fold.
Excess rate of any venous thromboembolism by age and sex groups, in the Covid-19 cohort (a) and following the first dose of Covid-19 vaccines (b). The scale of the ordinal axis differs by 50-fold.
Regarding vaccine-related differences, a significantly higher event rate of VTE was observed in individuals who received the Moderna vaccine than those who received the Pfizer vaccine. However, this rate was significantly lower in individuals who received the AstraZeneca vaccine.
Regarding event-related mortality, about 19% of individuals who developed unusual site VTE or VTE associated with TCP after vaccination died during the study period. Moreover, death occurred in 15% of individuals who developed VTE and TCP after vaccination.
Study significance
The study reveals that although COVID-19 vaccines are associated with an increased incidence rate of VTE events and TCP, the risk of these clinical events is significantly higher among COVID-19 patients. Moreover, men are more susceptible to thrombosis and/or TCP than women following vaccination.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.