A recent study from the University of Rochester reveals how the biodistribution kinetics and the affinity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for many different organs in the human body can be affected by antibodies targeting viral spike glycoprotein. The paper is currently available on the bioRxiv* preprint server while it undergoes peer review.
The ongoing coronavirus disease 2019 (COVID-19) is caused by the SARS-CoV-2, a virus that can enter host cells by using its spike glycoprotein to bind with the angiotensin-converting enzyme 2 (ACE2) receptor widely distributed in tissues and organs.
As a result, this can result in multiorgan failure in severe cases of the disease; nonetheless, it is not clear whether there is a differential multiorgan biodistribution and organ uptake in healthy young individuals usually presenting with asymptomatic or moderate COVID-19 symptoms.
Furthermore, for antibody treatments and vaccines that target the spike glycoprotein, it is unclear whether these reduce SARS-CoV-2 or spike glycoprotein multiorgan tropism equally. This is rather important, as mRNA vaccines release translated spike protein into interstitial fluid and blood, which is distributed to a plethora of organs in order to trigger an immune response.
This is the reason why a research group, led by Dr. Molly Brady from the Department of Neuroscience at the University of Rochester in the state of New York, decided to take a deep look into SARS-CoV-2 biodistribution kinetics and multiorgan tropism in order to get more conclusive answers.
Innovative use of dynamic imaging methods
In this study, the researchers have utilized the receptor-binding domain (RBD) of the spike glycoprotein as a SARS-CoV-2 viral surrogate in order to appraise its biodistribution and elimination in laboratory mice – primarily due to the fact that RBD structure has a key role for viral entry into host cells.
One of the key approaches to test the effect of anti-ACE2 and anti-spike glycoprotein antibodies on the distribution of spike glycoproteins has been in vivo dynamic imaging in regions that were known to be affected by COVID-19, associating signals with a predominant organ.
Finally, ex vivo organ spike glycoprotein distribution analyses were also pursued at the end of experiments in order to better understand which organ was mostly associated with SARS-CoV-2 spread. More specifically, organs such as lungs, liver, brain, lungs, spleen, kidneys and intestines were removed, washed and imaged – akin to in vivo imaging approach.
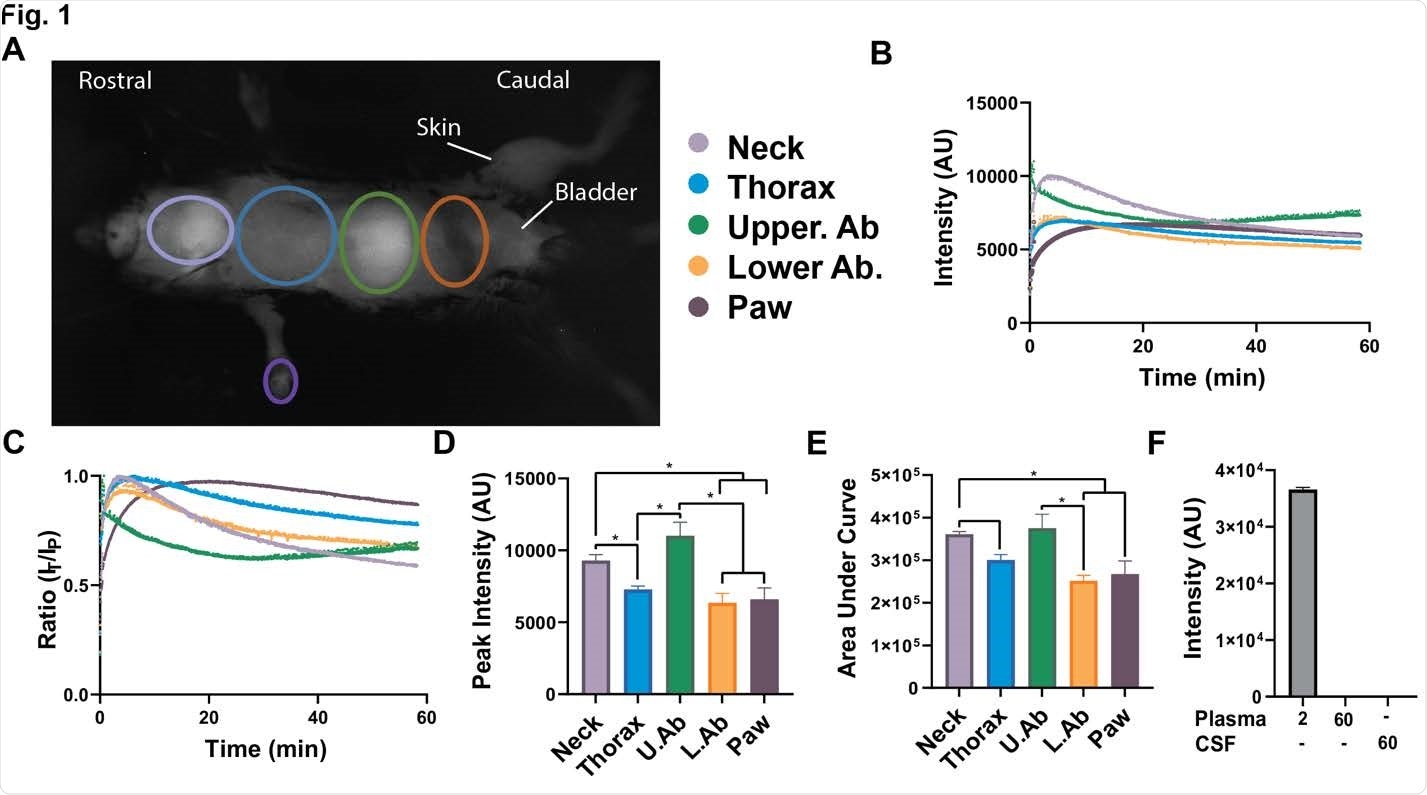
Body-wide biodistribution and slow elimination of a SP in mice A) External in vivo dynamic SP-NIRF image of a mouse after its intravenous injection (after a few minutes). Regions of interest (ROIs) selected for the analysis. B) Representative SP-NIRF intensity-time profile for each ROI over 60 min. C) Standardization of data by dividing intensities at each time point by the peak intensity (IT/IP ratio). D) Peak intensity for the ROIs. E) Area under the curve (AUC) for the ROIs. Values are mean ± SEM, N=5 young male mice (2-3 months old). F) Plasma intensities at 2 and at 60 min, and CSF levels at 60 min. Values are mean ± SEM, N=3 young male mice. AU (arbitrary units).
A snapshot of SARS-CoV-2 spike glycoprotein biodistribution
In short, this study has found a spike glycoprotein body-wide biodistribution which was followed by a slow regional elimination in 2-3 months old male mice, except for the liver, which actually showed a buildup of this biomarker.
Moreover, spike glycoprotein uptake was most abundant in the lungs, followed by kidney, heart and liver. Interestingly, it was not detected in the functional tissue of the brain (i.e., brain parenchyma) or cerebrospinal fluid.
In other words, vascular barriers of the brain were successful in restricting the entry of spike glycoprotein into brain parenchyma in young, healthy mice. While both anti-ACE2 and anti-spike antibodies showed suppressive traits, the latter was more effective in halting spike glycoprotein biodistribution and organ uptake.
Confirmed efficacy of therapies and vaccines
This study has demonstrated how differential spike glycoprotein organ uptake is primarily determined by ACE2 levels; however, additional research endeavors are needed in older mice and also in instances when systemic inflammation is present.
“Therapies that include passive immunity using anti-SARS-CoV-2 antibodies, and convalescent plasma which contains anti-SARS-CoV-2 antibodies, will be effective in reducing SARS-CoV-2 biodistribution and, thus, COVID-19 severity”, reinforce study authors in this bioRxiv paper.
Indirectly, by modeling the behavior of SARS-CoV-2 in a host, this study confirms that vaccines steered against the spike glycoprotein are a robust choice for neutralizing viral tissue distribution and minimizing the severity of this pervasive infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Brady, M. et al. (2021). Spike protein multiorgan tropism suppressed by antibodies targeting SARS-CoV-2. bioRxiv. https://doi.org/10.1101/2021.07.30.454520, https://www.biorxiv.org/content/10.1101/2021.07.30.454520v1
- Peer reviewed and published scientific report.
Brady, Molly, Conor McQuaid, Alexander Solorzano, Angelique Johnson, Abigail Combs, Chethana Venkatraman, Akib Rahman, et al. 2021. “Spike Protein Multiorgan Tropism Suppressed by Antibodies Targeting SARS-CoV-2.” Communications Biology 4 (1). https://doi.org/10.1038/s42003-021-02856-x. https://www.nature.com/articles/s42003-021-02856-x.