Coronaviruses that include severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), severe acute respiratory syndrome-coronavirus (SARS-CoV), and Middle-East respiratory syndrome -coronavirus (MERS-CoV) are known to cause severe respiratory disorders. These infections begin in the upper respiratory tract and progress rapidly to the lower respiratory tract.
With infection, symptoms vary from asymptomatic to fever and dry cough and finally to severe pneumonia. The severity of the disease also varies between different groups of people. Elderly patients have a higher risk of respiratory failure that can be fatal.
“Fatal respiratory failure due to lung damage in coronavirus infection is attributed to rapid virus replication and excessive immune responses,” say researchers from the University of Copenhagen in a new study.
The viral load within patients positively correlated with the severity of the disease. In addition, it was found through sputum and pharyngeal swab samples that the coronaviruses could reach a very high titer of 10^8-10^10 copies/ml within the upper respiratory tract of the affected patients.
This study proposes a mathematical framework to analyze the effect of the viruses on type I interferon (IFN-I) signaling, which is part of the innate immune response. IFN-I brings about natural killer cell activation, which in turn causes the activation of macrophages.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Battle between the virus and the immune system
All three coronaviruses were found to interfere with the IFN-I signaling pathway in the respiratory cells of the patients. IFN-I was initially produced by the infected cells and activated IFN-stimulated genes and natural killer cells (NK cells). The NK cells produced IFN-ϒ that led to the activation of macrophages.
Activation of macrophages was also brought about by Interleukin-6 (IL-6) that was secreted by the infected cells. The activated macrophages secreted a high amount of cytokines and chemokines that led to the activation of more macrophages.
“Thus, macrophages are capable of mediating an excessive potentially self-amplifying innate immune response by positive feedback if activated beyond a tipping point,” says the team.
The over-activated macrophages and inflammatory cytokines lead to the leakage of substrates from the blood into the respiratory tissue. Thus, the leading causes for mortality associated with coronavirus diseases are cytokine storm, unregulated production of cytokines, and high risk of multiple organ dysfunction even after the viruses are removed.
What did the study find?
The study involved a detailed mathematical model which included four variables; the activated NK cells, the viral load, the activated macrophages, and the monocyte-derived macrophages. The outcome of the viral infection was captured at four different stages; Chronic stage with prompt IFN-I signaling, Mild stage with normal IFN-I signaling, Severe disease with insensitive IFN-I signaling, and decrease in the severity of disease on treatment with IFN-I blockade.
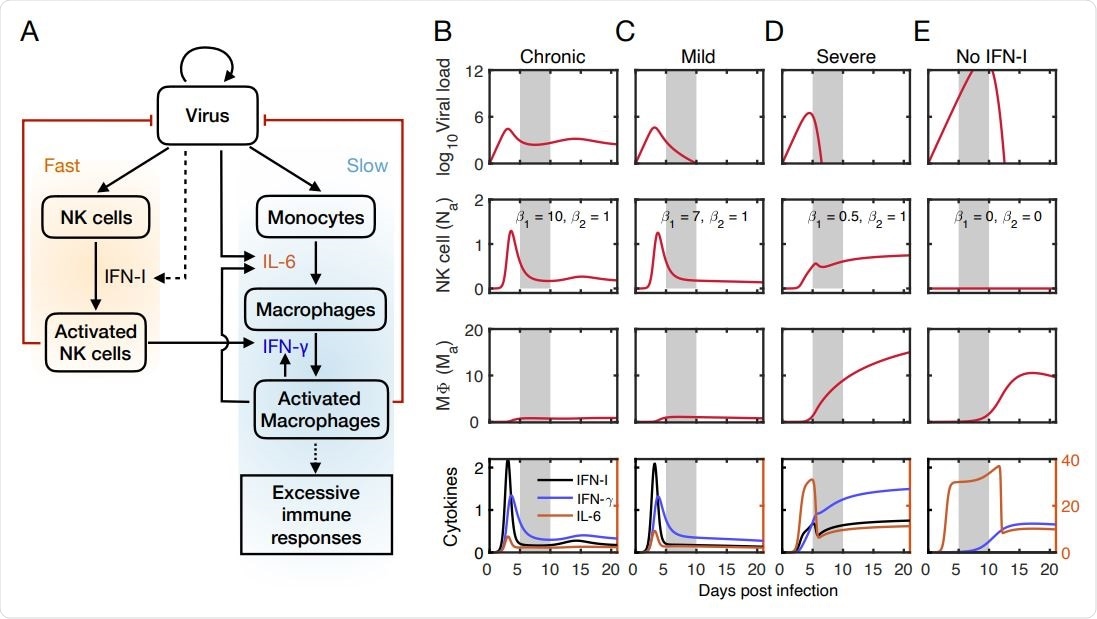
Mathematical model captures outcomes of viral infection at the early stage.
The model also predicts that high sensitivity to NK cell reaction prevents the viral load from reaching high numbers. In contrast, low sensitivity to NK cell reactions leads to high viral load and elevated activated macrophage levels. The host recovers only if the macrophage activation level is below a certain threshold during viral clearance.
The model also found that interactions between the immune system and the virus can lead to chronic patterns with recurrent disease. The chronic pattern is caused by NK cells due to a strong reaction with the virus that reduces viral load. This prevents macrophage accumulation that results in persistent viral load.
What did the authors conclude?
“Our model highlights the implications of the dysfunction of NK cells in the innate immunity caused by the insensitivity of IFN-I response to viral infection”, says the team.
The model discussed in this study had certain limitations. For example, it did not consider the anti-inflammatory cytokines secreted by macrophages that led to a reduction in recruitment of macrophages, the model considers only a single site infection, and data for virus or cytokine kinetics before the symptom offset was lacking.
“Our model gives a general way to think about how to alter the treatment of the patients who are at different stages when admitted to hospital. The protective and pathogenic roles of NK cells need to be delicately balanced when targeting IFN-I in combination with other therapies”, adds the team.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.