Researchers from the Tohoku Univerisity, Japan, and Christian Albrechts University Kiel, Germany, critically review the use of surfactant-coated nanoparticles in the fields of nanomedicine and food nanotechnology. The review, which was recently published in the International Journal of Nanomedicine, also discusses the current progress and challenges associated with surfactant-coated nanoparticle technology.
Surfactants are long-chain amphiphilic compounds that consist of both hydrophobic and hydrophilic groups in their chemical structure. These compounds can be classified according to the characteristic of the hydrophilic group, which includes four distinct subtypes. These include cationic, anionic, zwitterionic, and nonionic surfactants.
The properties of surfactants often depend on the hydrophilic-hydrophobic balance (HLB), which is a parameter that indicates the surfactant’s affinity for water and oil. The critical packing parameter (CPP), which is a parameter that predicts the surfactant’s self-assembly, also largely determines the structural characteristics of the surfactant.
Whereas cationic surfactants are considered the most toxic type of surfactant, nonionic surfactants are considered to be the least toxic. As a result, nonionic surfactants are used in a wide range of healthcare, cosmetic, and food products. In addition to the low toxicity profile of nonionic surfactants, these compounds are also associated with low production costs, high stability, and amphiphilic nature.
The reduced toxicity of nonionic surfactants is largely due to their hydrophilic groups that do not ionize in aqueous solutions. As a result, the micelle concentration of nonionic surfactants is often much lower as compared to ionic surfactants, thus reducing their toxicity profile.
Surfactant-coated nanoparticles
The surface structure of nanoparticles often determines their dispersity in water and aggregation dynamics. Functionalizing the organic or inorganic nanoparticle surface with surfactants stabilizes them, thus increasing their ability to disperse in water and speed of aggregation. These nanoparticles are called “surfactant-coated nanoparticles.”
Surfactant-coated nanoparticles in nanomedicine
Some of the common ways in which organic nanoparticles are used in nanomedicine are for the encapsulation of anticancer drugs, genes, and proteins for targeted delivery. The selective delivery of organic nanoparticles increases the therapeutic efficacy of drugs while simultaneously reducing unwanted side effects and protecting the encapsulated drug from premature degradation.
Within the field of nanomedicine, surfactants are often used to enhance the functionality of nanoparticles. Surfactants have been widely used to coat organic nanoparticles that are used for disease treatment purposes.
The most common surfactant that is used to prepare poly(lactic-co-glycolic acid) (PLGA) nanoparticles is polyvinyl alcohol (PVA). However, other surfactants that have been used to coat organic nanoparticles for biomedical purposes include sodium taurocholate and sodium cholate (SC).
Surfactants can also be used within nanomedicine to change the charge of the nanoparticle surface in order to enhance their intracellular localization. More specifically, cationic charged nanoparticles, as compared to anionic and nonionic charged nanoparticles, often exhibit a higher cellular uptake due the electrostatic attraction that arises between the nanoparticles and the negatively charged surface of cells. Some cationic surfactants that have been used to coat nanoparticles for this purpose include cetyltrimethylammonium bromide (CTAB) and didodecyldimethylammonium bromide (DDAB).
Despite their advantages for cellular uptake, cationic charged nanoparticles are more likely to alter the integrity of the cell membrane and cause damage to organelles including the mitochondria and lysosomes. In an effort to overcome the potentially toxic effects of these surfactants, nonionic surfactant-coated nanoparticles are associated with reduced side effects.
Taken together, the most widely used surfactant that is used to coat nanoparticles for biomedical purposes is polyethylene glycol (PEG). Despite its advantages, there are concerns regarding the accelerated blood clearance (ABC) phenomenon, which is an immune response that removes PEG-modified nanoparticles from the body. Research is currently underway to enhance the functions of the PEG-modified nanoparticles while simultaneously weakening the ABC phenomenon.
Surfactant-coated nanoparticles in food nanotechnology
The application of nanotechnology into the food industry has significantly increased over the past several years as a result of its utility in improving the properties of existing food products. For example, the encapsulation of food ingredients into nanoparticles has extended the shelf life of these products while simultaneously reducing their degradation by the digestive system.
Although some nanoparticles are able to overcome the harsh environment of the digestive tract, which includes various digestive enzymes and the highly acidic stomach, there is still room for improvement to ensure the complete absorption of encapsulated nutrients. To this end, surfactant-coated nanoparticles have been developed to not only overcome some of the aforementioned environmental challenges of the gastrointestinal tract but also penetrate the mucus barrier within the small intestines in order to ensure the absorption of their contents by intestinal cells.
Food nanosensing is another application of nanoparticles within the food industry, which utilizes inorganic nanoparticles to detect a wide range of characteristics in food products. More specifically, nanoparticles are used to sense the presence of adulterants, artificial ingredients, bacterial toxins and other pathogens. Taken together, these nanoparticles are important for ensuring the safety and quality of food products before they reach the consumer.
Like many other aspects of food nanotechnology, surfactants are also used in food nanosensing to further enhance the properties of these nanoparticles. Magnetite nanoparticles coated with cationic surfactants, for example, are associated with greater limits of detection. Surfactants have also been applied to the surface of silver nanoparticles, which are the most widely used type of nanoparticles in the food industry, to prevent food products from dehydration and microbial spoilage.
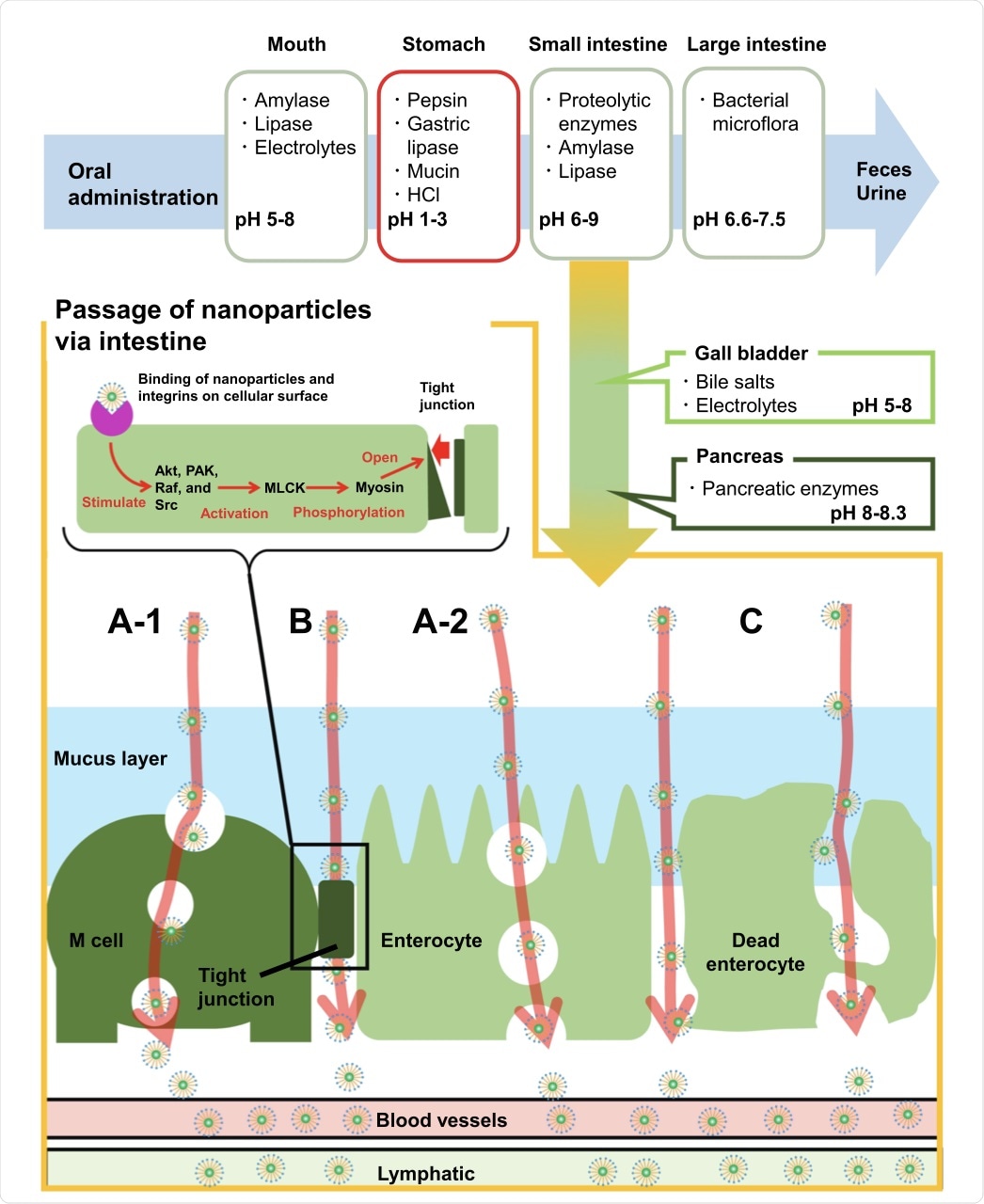 The digestive stages after oral administration and the mechanisms of in vivo uptake of surfactant-coated nanoparticles through the small intestine. Notes: (A-1) Transcellular route, through the M cells. (A-2) Transcellular route, through the enterocyte. (B) Paracellular route. (C) Persorption route.
The digestive stages after oral administration and the mechanisms of in vivo uptake of surfactant-coated nanoparticles through the small intestine. Notes: (A-1) Transcellular route, through the M cells. (A-2) Transcellular route, through the enterocyte. (B) Paracellular route. (C) Persorption route.
Conclusions
As nanotechnology continues to become a more common component of both the medical and food industries, surfactants will inevitably play an important role in enhancing the functions of nanoparticles. While further work must be conducted in order to ensure the safety of both nanoparticles and the surfactants that are used to coat their surfaces, the combination of these two technologies has a promising future.