A team of international scientists has recently demonstrated the usefulness of cetylpyridinium chloride-containing mouthwashes in inhibiting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and reducing the risk of viral transmission. The study is currently available on the bioRxiv* preprint server.
Due to the presence of angiotensin-converting enzyme 2 (ACE2) in the oral gingival epithelium and salivary glands, the human oral cavity may act as a reservoir for SARS-CoV-2. The ACE2 present on the host cell membrane acts as the primary entry receptor for SARS-CoV-2. Evidence indicates that the saliva of SARS-CoV-2-infected individuals contains high amounts of viral RNA and that aerosols formed from the saliva can act as a potential vector for viral transmission.
Several health organizations have advised using mouthwashes during dentistry procedures as a measure to inhibit SARS-CoV-2 transmission. Bioactive ingredients present in mouthwashes, including dequalinium chloride, benzalkonium chloride, cetylpyridinium chloride, and chlorhexidine, have been found to inhibit pathogens by charge-mediated attraction and destabilization of the lipid envelope.
In the current study, the scientists have investigated in vitro antiviral efficacy of mouthwashes containing cetylpyridinium chloride (0.07%) or chlorhexidine digluconate (0.2%) against SARS-CoV-2 and its variants, including B.1.1.7, B.1.351, and P1. Moreover, in a separate set of in vitro experiments, they have determined the antiviral efficacy of cetylpyridinium chloride-containing mouthwash in the presence of human saliva to investigate whether salivary components can deactivate the active agent.
To measure viral titers, they conducted a plaque assay after 30 seconds of mouthwash exposure. They tested different mouthwash formulations including cetylpyridinium chloride with flavor, cetylpyridinium chloride with flavor and herbal mix, and chlorhexidine digluconate with flavor. As positive and negative controls, they have used 70% ethanol and water, respectively.
Important observations
The plaque assay findings revealed that both formulations of cetylpyridinium chloride-containing mouthwash are capable of inhibiting SARS-CoV-2 by 99.99%. After 30 seconds of exposure to these formulations resulted in a viral titer value below the detection limit. In contrast, chlorhexidine digluconate-containing mouthwash showed significantly lower efficacy in inhibiting SARS-CoV-2.
Furthermore, both formulations of cetylpyridinium chloride-containing mouthwash exhibited high efficacy in reducing the titers of B.1.1.7, B.1.351, and P1 below the detection limit. However, no such reduction was observed for chlorhexidine digluconate-containing mouthwash.
Functionality of mouthwash in presence of human saliva
Scientists first tested whether mouthwash formulations remain effective in the presence of saliva by testing the endogenous antiviral efficacy of saliva against SARS-CoV-2. However, they could not observe any reduction in viral titers after 5 minutes of exposure to saliva. This indicates that human saliva alone does not have any intrinsic antiviral activity.
Afterward, they tested whether salivary components can alter the anti-SARS-CoV-2 efficacy of cetylpyridinium chloride. The findings revealed that cetylpyridinium chloride-containing mouthwash is capable of completely inhibiting the virus even in presence of saliva.
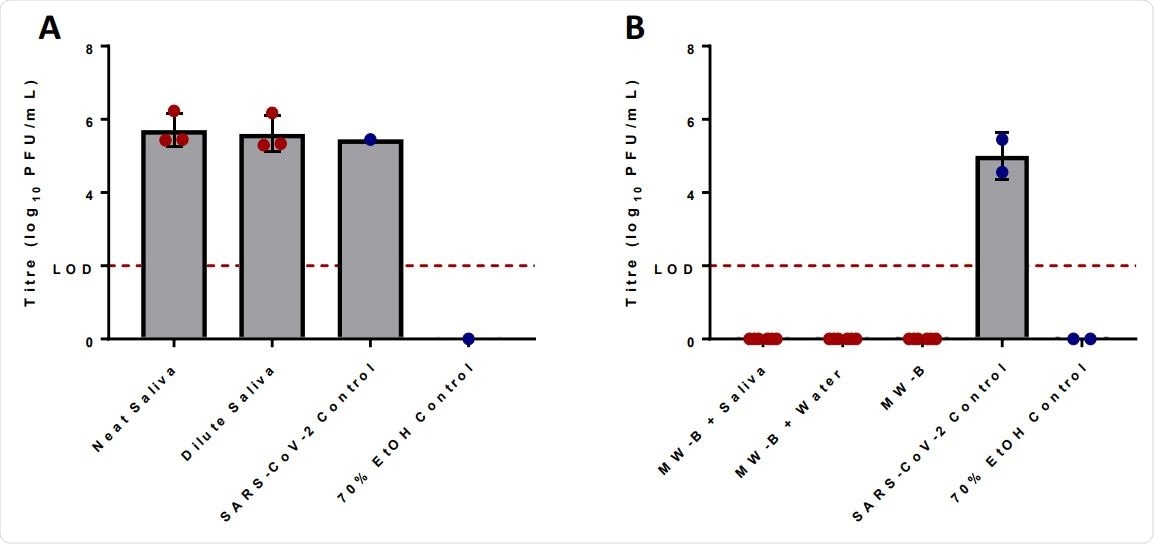
Irradiated human saliva has no effect upon the viral titer of SARS-CoV-2 as compared to the water control after incubation with inoculum for 5 minutes. Neat saliva had a ratio of 8 parts water to 1-part irradiated human saliva to 1-part virus inoculum, while dilute saliva had a ratio 9 parts irradiated human saliva to 1-part virus inoculum (A). Human saliva does not inhibit the antiviral activity of mouthwash formulas proven to reduce the titer of SARS-CoV-2 (B). MW-B was able to reduce viral titer to below the LODboth in the presence of irradiated human saliva and without. Human saliva was added in a ratio of 8 parts MW-B to 1-part irradiated human saliva to 1-part virus inoculum. Limit of detection (LOD) (2.0log10 PFU/mL) is shown across both graphs with a dotted red line. Error bars represent standard deviation, while red dots are experimental data values and blue dots control values.
Study significance
The study highlights the potential of cetylpyridinium chloride-containing mouthwashes in inhibiting SARS-CoV-2 and its variants. Regular use of such low-cost mouthwashes as a good oral hygiene practice could potentially reduce the risk of viral transmission to others, as well as through the respiratory tract of an infected person.
Anti-SARS-CoV-2 effectiveness of cetylpyridinium chloride-containing mouthwashes has also been reported in other studies. In addition, human clinical trials are reporting that rinsing the oral cavity with cetylpyridinium chloride-containing mouthwash can effectively reduce the amount of SARS-CoV-2 in saliva for several hours.
Some recent studies have shown that plaque build-up in the oral cavity due to poor hygiene, and subsequent gum infection can increase the risk of viral entry via the oral gingival sulcus and periodontal pockets, leading to respiratory and systemic SARS-CoV-2 infection. Based on the current study findings, such risk can be reduced by regular use of cetylpyridinium chloride-containing mouthwashes.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Anderson ER. 2021. Virucidal activity of CPC-containing oral rinses against SARS-CoV-2 variants and are active in the presence of human saliva, bioRxiv, https://doi.org/10.1101/2021.08.05.455040, https://www.biorxiv.org/content/10.1101/2021.08.05.455040v2
- Peer reviewed and published scientific report.
Anderson, Enyia R., Edward I. Patterson, Siobhan Richards, Ana K. Pitol, Thomas Edwards, Dominic Wooding, Kate Buist, et al. 2022. “CPC-Containing Oral Rinses Inactivate SARS-CoV-2 Variants and Are Active in the Presence of Human Saliva.” Journal of Medical Microbiology 71 (2). https://doi.org/10.1099/jmm.0.001508. https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.001508.