COVID-19 vaccines have led to drastic decreases in the number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases and mortality in the US; however, most of the patients in the vaccine trials did not have chronic underlying comorbidities, which is a major risk factor for COVID-19 morbidity and mortality. In addition, host factors in such high-risk patients may cause reduced responsiveness to COVID-19 vaccines and lead to “breakthrough” COVID cases.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Using real-world data to evaluate if chronic conditions cause reduced antibody response to COVID-19 mRNA vaccines
High-risk individuals with a decreased immune response to the COVID-19 vaccines may stop adhering to protective measures once masking and distancing mandates are lifted without knowing their potential vulnerability.
According to a recent report, fully vaccinated health care workers had breakthrough infections and lower neutralizing antibody titers during the peri-infection period compared to those in matched uninfected controls.
Also, a recent study reported that 46% of transplant patients had no antibody response after two doses of mRNA vaccines. However, no studies so far have investigated the effects of chronic medical conditions on antibody response to the vaccines.
A recent study in Colorado used real-world data to evaluate if individuals with chronic conditions have reduced antibody response to COVID-19 mRNA vaccines. The study investigated whether some medical conditions may impact antibody response to the mRNA vaccines. This work is available on the medRxiv* preprint server. The study participants were from patients in National Jewish Health, Colorado - a pulmonary specialty outpatient clinic.
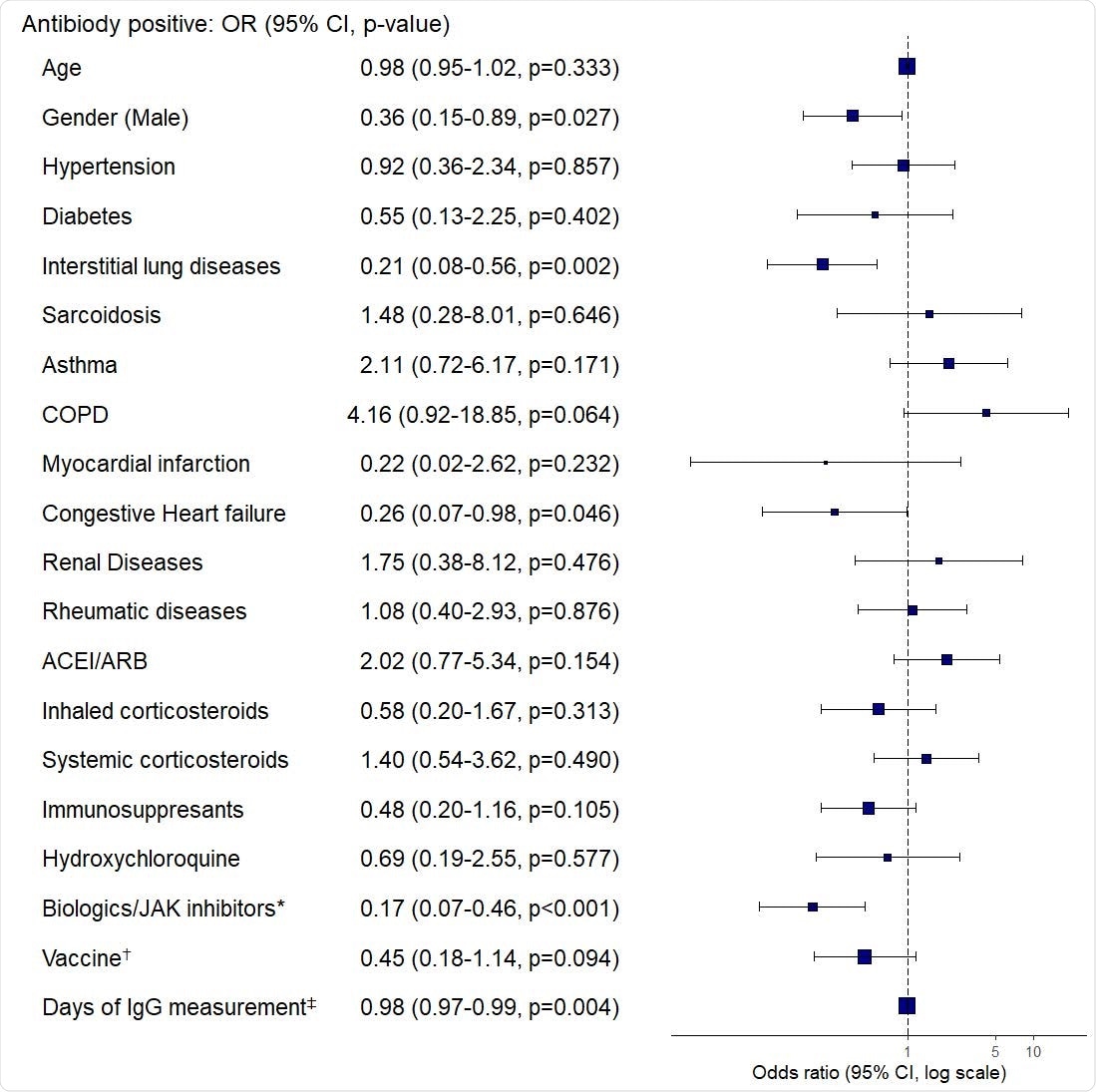
Multivariate logistic regression of the association between antibody response and clinical characteristics. OR: odds ratio; CI: confidence interval; COPD: chronic obstructive pulmonary disease; ACE/ARB: angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers. *Biologics: Anti- IL-5, -IL-6, -IL-12/23, -IL-17, -IgE, -CD20, and -TNF-α inhibitors †BNT162b2 (Pfizer-BioNTech) compared to mRNA-1273 (Moderna) ‡Days after 2nd vaccine dose.
Interstitial lung disease is an independent risk factor for reduced antibody response
The work highlights that 26% of the patients (n=226) with spike protein ab measured 14 days after the second vaccine dose had negative spike protein ab testing. The researchers found that interstitial lung disease (ILD) is an independent risk factor for reduced antibody response.
While the precise antibody level that offers protection against SARS-CoV-2 is not known and there may be non-B cell-mediated protection such as T cell-mediated protection, this study raises concerns that COVID-19 vaccination may not lead to protective immunity in all populations and may have profound implications for some high-risk individuals once masking and distancing strategies are lifted.
“Our study raises concerns that SARS-CoV-2 vaccination may not result in protective immunity in all populations and may have implications for some as masking and distancing strategies are abandoned.”
Results show that CHF, ILD, and rituximab use are independent risk factors for decreased antibody response
The findings of this study raise concerns that SARS-CoV-2 vaccination may not lead to protective immunity in patients with chronic underlying conditions. It is very crucial to recognize that vaccination may not necessarily offer protective immunity to all individuals, particularly in people with certain chronic conditions and medication use, at least as measured by commercial spike protein antibody tests.
In this study, 79% of the patients had chronic pulmonary diseases and 26% of them had no antibody detected over 14 days after the second dose of mRNA vaccines. While these data demonstrate that all these vaccinated individuals may be at risk for reduced antibody production, it was found that CHF, ILD, and biologic/JAK inhibitor use (particularly rituximab) are independent risk factors for decreased antibody response.
According to the authors, they have not yet observed the occurrence of COVID-19 in these patients who have negative antibody responses. However, due to the relatively low prevalence of COVID-19 in the Colorado region, the relatively short time since vaccine administration, lack of strict follow-up to assess infections, and the recommendations to continue mask-wearing and other precautions, this outcome is not surprising.
“Further studies of immunologic response (including neutralizing antibodies and other markers of B cell and T cell response) and protection in vulnerable populations are needed to define ongoing COVID-19 risk and inform recommendations regarding additional vaccination and behaviors to mitigate risk.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Impaired SARS-CoV-2 mRNA vaccine antibody response in chronic medical conditions: a real-world data analysis Shu-Yi Liao, Anthony N. Gerber, Pearlanne Zelarney, Barry Make, Michael E. Wechsler, medRxiv, 2021.08.03.21261483; doi: https://doi.org/10.1101/2021.08.03.21261483, https://www.medrxiv.org/content/10.1101/2021.08.03.21261483v1
- Peer reviewed and published scientific report.
Liao, Shu-Yi, Anthony N. Gerber, Pearlanne Zelarney, Barry Make, and Michael E. Wechsler. 2022. “Impaired SARS-CoV-2 MRNA Vaccine Antibody Response in Chronic Medical Conditions: A Real-World Analysis.” CHEST 161 (6): 1490–93. https://doi.org/10.1016/j.chest.2021.12.654. https://journal.chestnet.org/article/S0012-3692(22)00015-0/fulltext.