The coronavirus disease 2019 (COVID-19) pandemic has infected over 240 million people worldwide and caused the deaths of over 4.3 million. The early and unprecedented investment into vaccines led to the emergency authorization of two mRNA vaccines including the BNT162b2 from Pfizer/BioNTech and mRNA-1273 from Moderna. At the time of their approval, both of these vaccines showed 94-95% efficacy against symptomatic COVID-19 within 7-14 days of the second dose.
As of August 10, 2021, only about 59% of the United States population has been vaccinated. Further, only 30.2% of the world population has received at least one dose of a COVID-19 vaccine. Taken together, the slow rate of vaccinations worldwide has allowed other SARS-CoV-2 variants to emerge, some of which are associated with increased resistance to neutralization by antibodies elicited by natural infection or vaccination.
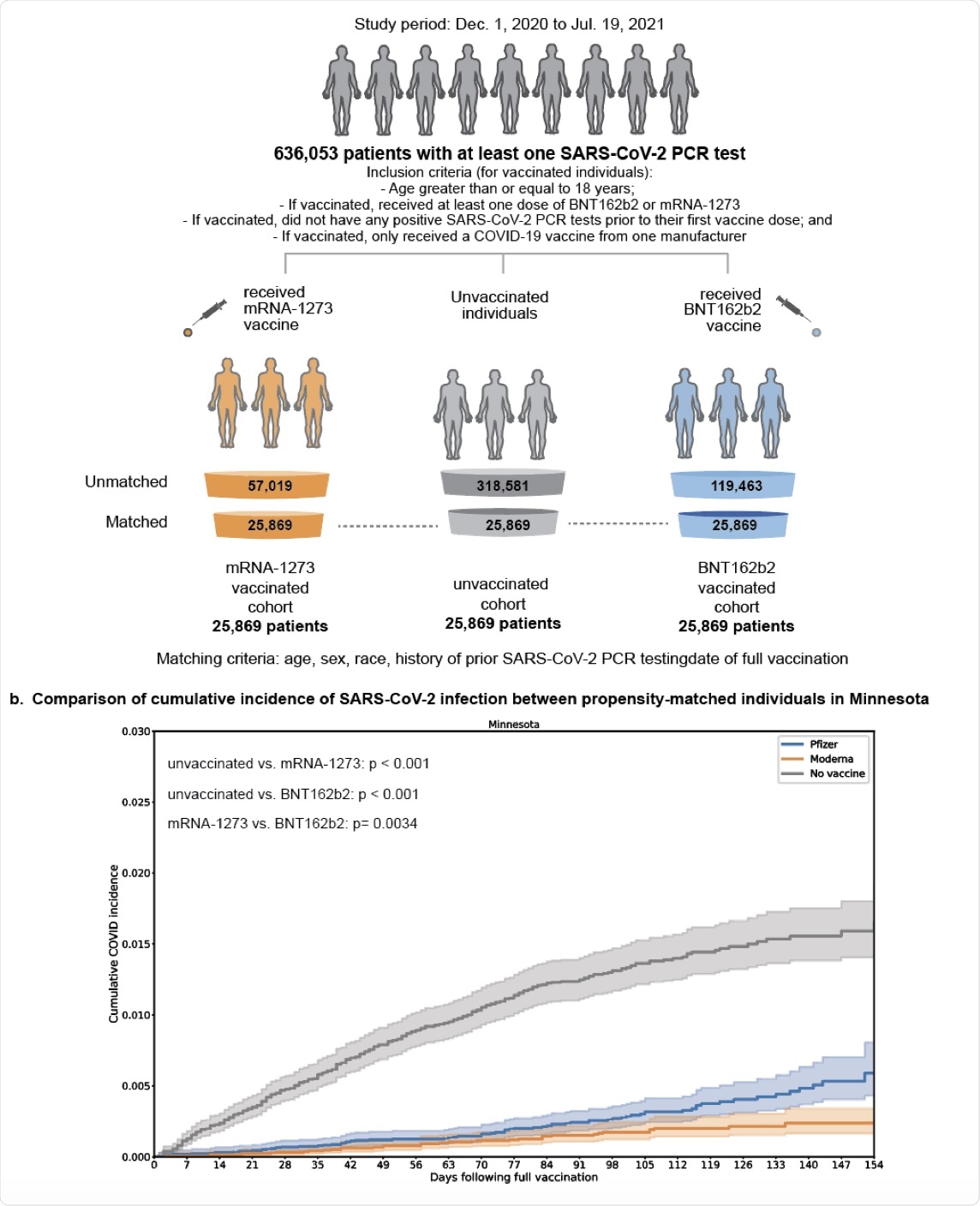 Study Overview. (A) Derivation of matched vaccinated and unvaccinated cohorts to compare the effectiveness of the mRNA COVID-19 vaccines mRNA1273 and BNT162b2. The matching process yielded 25,689 triples of individuals (one unvaccinated, one vaccinated with mRNA-1273, one vaccinated with BNT162b2) from Minnesota who were matched on the basis of age, sex, race, ethnicity, history of prior SARS-CoV-2 PCR testing, and date of vaccination. (B) With the cohorts described in (A), we assessed the overall effectiveness of each vaccine by comparing the cumulative incidence of infection in each vaccinated cohort compared to the matched unvaccinated cohort. We also assessed the relative effectiveness of each vaccine (i.e., incidence rate of infection in the mRNA-1273 cohort compared to the BNT162b2 cohort).
Study Overview. (A) Derivation of matched vaccinated and unvaccinated cohorts to compare the effectiveness of the mRNA COVID-19 vaccines mRNA1273 and BNT162b2. The matching process yielded 25,689 triples of individuals (one unvaccinated, one vaccinated with mRNA-1273, one vaccinated with BNT162b2) from Minnesota who were matched on the basis of age, sex, race, ethnicity, history of prior SARS-CoV-2 PCR testing, and date of vaccination. (B) With the cohorts described in (A), we assessed the overall effectiveness of each vaccine by comparing the cumulative incidence of infection in each vaccinated cohort compared to the matched unvaccinated cohort. We also assessed the relative effectiveness of each vaccine (i.e., incidence rate of infection in the mRNA-1273 cohort compared to the BNT162b2 cohort).
What were the findings?
The current study showed that between January and July 2021, in the American state of Minnesota, the mRNA-1273 and BNT162b2 vaccines were 86% and 76% effective, respectively, in preventing SARS-CoV-2 infection, beginning at 14 or more days from the second dose.
Completed vaccination with the BNT162b2 and mRNA-1273 reduced the risk of hospitalization with COVID-19 by 85% and 92%, respectively. Additionally, admission to the intensive care unit (ICU) by these two vaccines was reduced by 93% and 87%, respectively. Mortality with the two vaccines was zero.
The researchers found that as COVID-19 cases increased in the state throughout July 2021, the mRNA-1273 vaccine showed 76% effectiveness, whereas the efficacy of the BNT162b2 vaccine against SARS-Cov-2 infection was only 42%. It is important to note that these figures reflect SARS-CoV-2 infection rates alone, rather than hospitalization rates in vaccinated individuals.
Breakthrough infections were much lower in the mRNA-1273 cohort as compared to the BNT162b2 cohort. The incidence rate was 0.017 vs 0.031, respectively, for an incidence rate ratio (IRR) of 0.56. Since the risk of infection was similar at baseline for both variants, this finding is significant.
However, both vaccines were equally effective at reducing the rates of hospitalization and of ICU admission, at IRRs of 0.57 and 0.53, respectively, with no deaths in either cohort.
The scientists then extended their observations to breakthrough infections from other states covered by the Mayo Clinic Health System, such as Wisconsin, Arizona, Florida, and Iowa. For the most part, full vaccination with mRNA-1273 reduced the risk of breakthrough infections, with IRRs of 0.29 to 0.52 in these states; except for Iowa, where it was zero.
When all states are clubbed, the IRR is 0.50, thereby indicating that the risk of breakthrough infections is halved by the mRNA-1273 vaccine, with the greatest risk reduction being seen in July, at 0.44. At this point, the dominant SARS-CoV-2 circulating strain in the United States was the Delta variant.
In Florida in July 2021, the risk of infection was reduced by 60% after mRNA-1273. Again, hospitalizations were halved after mRNA-1273 vaccination as compared to BNT162b2, while ICU admission rates were similar with both.
The risk of complications was similar between both vaccines. At 21 days, the risk for all of the aforementioned events ranging from hospitalization to mortality was comparable.
What are the implications?
Breakthrough infections have diminished public confidence in the COVID-19 vaccines. This is coupled with laboratory evidence that vaccine-induced antisera failed to neutralize the newer SARS-CoV-2 variants of concern (VoCs), such as the Delta variant, as effectively as they did with the older strains.
The current study demonstrates a continuing high vaccine efficacy against SARS-CoV-2 infection and against severe COVID-19. Comparisons between the two showed that breakthrough infections were only half as frequent in those who were vaccinated with both the mRNA-1273 and BNT162b2 vaccines in several states.
A previous study has shown similar findings, with BNT162b2 preventing 88% of symptomatic infection with the Delta variant as compared to 94% with the Alpha variant. Both of these observations are indicative of the high efficacy of the BNT162b2 vaccine.
Secondly, both vaccines, particularly the BNT162b2 vaccine, showed reduced effectiveness in July when the prevalence of the Delta variant was rising. Several additional factors may have contributed to this, other than the loss of neutralizing activity.
For instance, each mRNA-1273 dose provides three times more copies of spike-encoding mRNA than BNT162b2, which may induce a more robust immune response. The two vaccines also use different lipid nanoparticles for encapsulation.
Both vaccines showed similar efficacy in preventing COVID-19-related complications. Overall, both vaccines continue to fulfill their original goal in reducing the burden of symptomatic disease, hospitalization, and death related to SARS-CoV-2 infection.
It is incorrect to consider these vaccines ineffective because they are not 100% effective in preventing SARS-CoV-2 infection. The reality is that very few vaccines are completely effective, especially for viruses that cause a significant proportion of asymptomatic infections. The flu shots administered annually in the United States, for example, are effective in reducing between 20-60% of infections.
Despite the localized nature of the study cohorts, the current study suggests that, in the real world, one vaccine offers greater protection than the other, and both were more effective earlier in the pandemic as compared to right now. With this data in mind, the authors insist that these mRNA vaccines are strongly protective against severe and critical COVID-19 and against SARS-CoV-2 infection.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Puranik, A., Lenehan, P. J., Silvert, E., et al. (2021). Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv. doi:10.1101/2021.08.06.21261707. https://www.medrxiv.org/content/10.1101/2021.08.06.21261707v1.
- Peer reviewed and published scientific report.
Puranik, Arjun, Patrick J Lenehan, John C O’Horo, Colin Pawlowski, Abinash Virk, Melanie D Swift, Walter Kremers, et al. 2022. “Durability Analysis of the Highly Effective MRNA-1273 Vaccine against COVID-19.” Edited by Karen E Nelson. PNAS Nexus 1 (2). https://doi.org/10.1093/pnasnexus/pgac058. https://academic.oup.com/pnasnexus/article/1/2/pgac058/6590065.