The coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evolved into a global pandemic destroying lives and economies. COVID-19 is not curable, so vaccines are the only safeguard during this pandemic.
Presently, several vaccines that are effective against COVID-19 are available. These vaccines have been found to be very effective in the general population. However, there is not enough evidence to show the extent of protection they provide in immunocompromised patients.
Effect of COVID-19 vaccines in immunocompromised patients
Vaccines are known to have a reduced immune response in immunocompromised patients. A number of studies have examined the effects of COVID-19 vaccines in patients suffering from autoimmune diseases, cancer, hemodialysis patients, patients on immunosuppressors like organ transplant recipients.
As each condition impacts the immune system differently, the effect of COVID-19 vaccines was found to vary depending on the condition that was studied. It becomes essential to evaluate the extent of protection these vaccines provide in the context of all immunocompromised conditions. This will help design an effective dosage and immunization schedule suitable for patients suffering from each condition.
COVID-19 vaccines have not been studied extensively in immunocompromised patients infected with the Human Immunodeficiency Virus (HIV). Considering these aspects, scientists from the University of Montreal in Canada have attempted to study the effect of a (messenger RNA) mRNA COVID -19 vaccine in HIV-positive (HIV+) patients. The research is posted to the bioRxiv* preprint server while awaiting peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Study on COVID-19 vaccine’s immunogenicity in HIV+ patients
Cluster of designation 4 (CD4) T cells are a type of white blood cells that play an essential role in the body’s fight against infections. In HIV+ patients, there is progressive destruction of CD4T cells, leading to a weakened immune system.
Earlier studies show that patients suffering from HIV show reduced immune response to specific vaccines depending on their CD4T cell count. The present study was performed to determine:
- mRNA based COVID-19 vaccine’s immunogenicity (ability to elicit an immune response) in HIV+ patients
- Effect of CD4T cell counts on the immune response elicited by the vaccine
The receptor-binding domain (RBD) on the SARS-CoV-2 virus plays a vital role in facilitating the entry of the virus into cells resulting in infection. Therefore, the measure of the antibodies produced by the body against the RBD domain ( Anti-RBD IgG response) will help evaluate the immune response to the vaccine. The present study evaluated the COVID-19 vaccine’s immunogenicity in HIV+ patients and controls by measuring the Anti-RBD IgG response.
How was the study performed?
The study was conducted on HIV+ patients on antiretroviral therapy and the patients were stratified according to their CD4T cell counts. The HIV+ patients received the Moderna mRNA-1273 vaccine. The control group consisted of HIV-negative (HIV-) health care workers who received the Pfizer BNT162b2 vaccine.
Immunogenicity to the mRNA COVID-19 vaccines in both the groups were measured by evaluating the Anti-RBD IgG response using enzyme-linked immunosorbent serum assay (ELISA) at different time points (baseline, between 3 and 4 weeks after the first dose).
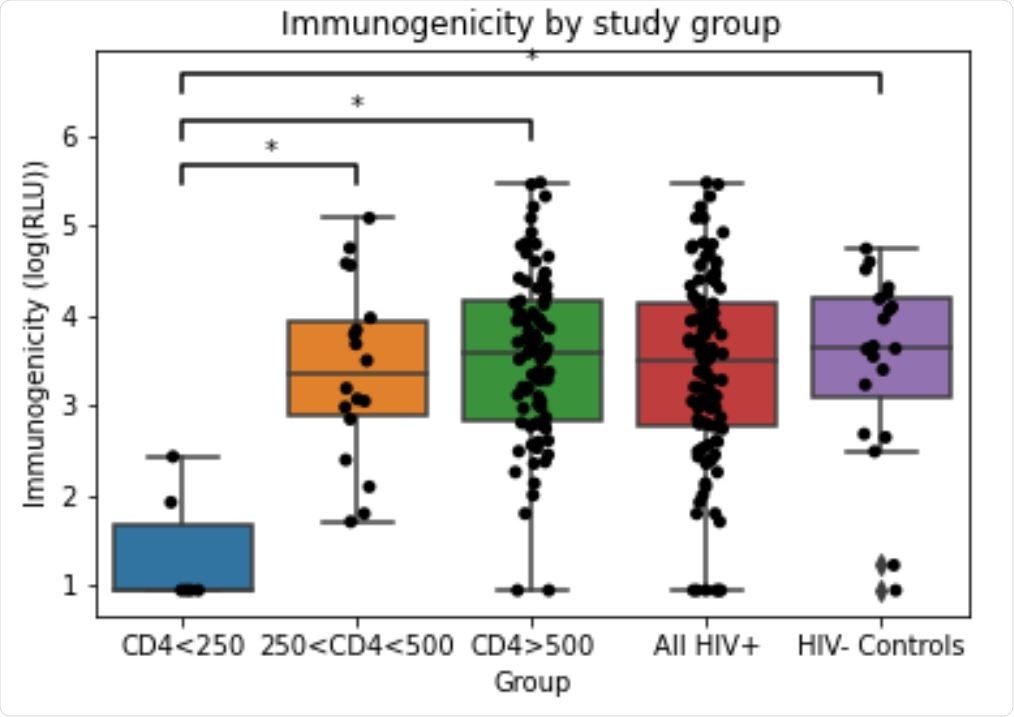
Immunogenicity in each study group. Immunogenicity (anti-RBD IgG response) was measured by ELISA and reported in RLU (relative luminescence units). RLU values log transformed for analysis. Statistically significant mean differences are denoted by * (Tukey test, p<0.001)
COVID-19 vaccine’s immunogenicity is reduced in HIV+ patients with less CD4T cell count
The results from the study reveal that between weeks 3 and 4 after the first mRNA COVID-19 vaccine dose:
- The vaccine immunogenicity in the unstratified HIV+ patient group is similar to the control group.
- Further, the vaccine immunogenicity in the control group and the patient groups stratified based on their CD4T cell counts was also compared. The results from this analysis revealed that the HIV+ patient groups with CD4T cell counts < 250 cells/mm3, showed statistically significant reduced response to the vaccine when compared to the control and other groups.
- The study also found a weak but statistically significant correlation between age and vaccine immunogenicity in HIV+ patients. The immunogenicity of the COVID-19 vaccine decreases in HIV-infected patients as their age increases. This is an exciting finding that was not observed in previous studies with HIV patients.
How to improve the effect of COVID-19 vaccines in HIV+ patients?
HIV+ patients with CD4T cell counts > 250 cells /mm3 show a similar immune response to the HIV- controls to mRNA-based COVID-19 vaccines. However, the immune response to the vaccine is reduced in HIV-infected patients who have CD4T cell counts < 250 cells / mm3. Based on these preliminary findings, it can be suggested that:
- During vaccinations, the HIV+ patients with CD4T cell count < 250 cells/mm3 should be identified.
- The identified patients should be recommended for a subsequent booster dose or their dose modified to enhance the protective effect of COVID-19 vaccines.
The scientists are further planning to follow the patients for one year to arrive at conclusive evidence.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Sources:
Journal references:
- Preliminary scientific report.
Covid-19 vaccine immunogenicity in people living with HIV-1 Lauriane Nault, Lorie Marchitto, Guillaume Goyette, Daniel Tremblay-Sher, Claude Fortin, Valérie Martel-Laferrière, Benoît Trottier, Jonathan Richard, Madeleine Durand, Daniel Kaufmann, Andrés Finzi, Cécile Tremblay bioRxiv 2021.08.13.456258; doi: https://doi.org/10.1101/2021.08.13.456258, https://www.biorxiv.org/content/10.1101/2021.08.13.456258v1
- Peer reviewed and published scientific report.
Nault, Lauriane, Lorie Marchitto, Guillaume Goyette, Daniel Tremblay-Sher, Claude Fortin, Valérie Martel-Laferrière, Benoît Trottier, et al. 2022. “Covid-19 Vaccine Immunogenicity in People Living with HIV-1.” Vaccine, May. https://doi.org/10.1016/j.vaccine.2022.04.090. https://www.sciencedirect.com/science/article/pii/S0264410X22005436?via%3Dihub.