At the end of February 2021, several cases of thromboses and thrombocytopenia were reported after individuals had been vaccinated against the coronavirus disease 2019 (COVID-19) with the ChAdOx1-S vaccine developed by Oxford-AstraZeneca. These reactions have been coupled into a syndrome known as vaccine-induced immune thrombocytic thrombocytopenia (VITT).
The thromboses identified in patients post-vaccination with the ChAdOx1-S vaccine were found in unusual sites including the cerebral venous sinuses, as well as the mesenteric and portal veins. The likely cause of VITT is an autoimmune response to platelet factor 4 (PF4) in the absence of heparin.
About the study
The objective of the current study was to quantify associations of vaccination with ChAdOx1-S and BNT162b2 with major arterial, venous, and thrombocytopenic events. This cohort study was conducted using electronic health records, with follow-up from December 8, 2020, to March 18, 2021. All participants in this study were adults over the age of 18 who were registered within England’s National Health Service (NHS) general practice.
The major outcome was measured in terms of incidence, incidence rate, and hazard ratios (HRs) for major arterial, venous, and thrombocytopenic that occurred between 1 and 28 days of the patients receiving the first dose of either the ChAdOx1-S or BNT162b2 vaccines. The observations were then analyzed separately for two age groups, which included those under the age of 70 and those older than 70. These groups were also arranged according to their sex, underlying comorbidities, social factors, and demographic details.
Study findings
By March 18, 2021, a total of 21,193,814 adults had received their first vaccination, of which 8,712,477 received BNT162b2 and 12,481,337 received ChAdOx1-S.
When calculating the risks of thrombotic events for every 100,000 participants between December 8, 2020, and March 18, 2021, thrombosis, any arterial thrombosis, and thrombocytopenia had a risk of 45.3, 189, and 4.2, respectively. Notably, the risks of both venous and arterial thromboses were higher in individuals who were older and had co-morbidities, while the risk varied significantly according to the individual’s ethnicity.
Patients who had a previous deep vein thrombosis (DVT) or pulmonary embolism (PE), thrombophilia, or took oral anticoagulants had a higher risk of venous thrombosis. Comparatively, individuals with a history of stroke, myocardial infarction (MI), or were taking antiplatelet medication had a higher risk of arterial thrombosis.
The hazard ratios of vaccination and pre-vaccination for the age group under 70 years and above 70 years was found to be 0.97 and 0.58, respectively, for venous thromboses in individuals who received the ChAdOx1-S vaccine. Comparatively, the risk for arterial thromboses was found to be 0.90 and 0.76 for arterial thromboses for under 70 and above 70 respectively.
The corresponding hazard ratios for the BNT162b2 vaccine were 0.81 and 0.57 for venous thromboses. Similar testing was run for arterial thromboses, which led to the finding that the ratio was 0.94 and 0.72 for age groups under 70 years and above 70 years, respectively.
The hazard ratios for thrombotic events were significantly increased for young age groups for venous thromboses after the ChAdOx1-S vaccine, as well as for arterial thromboses after the injection of both the vaccines.
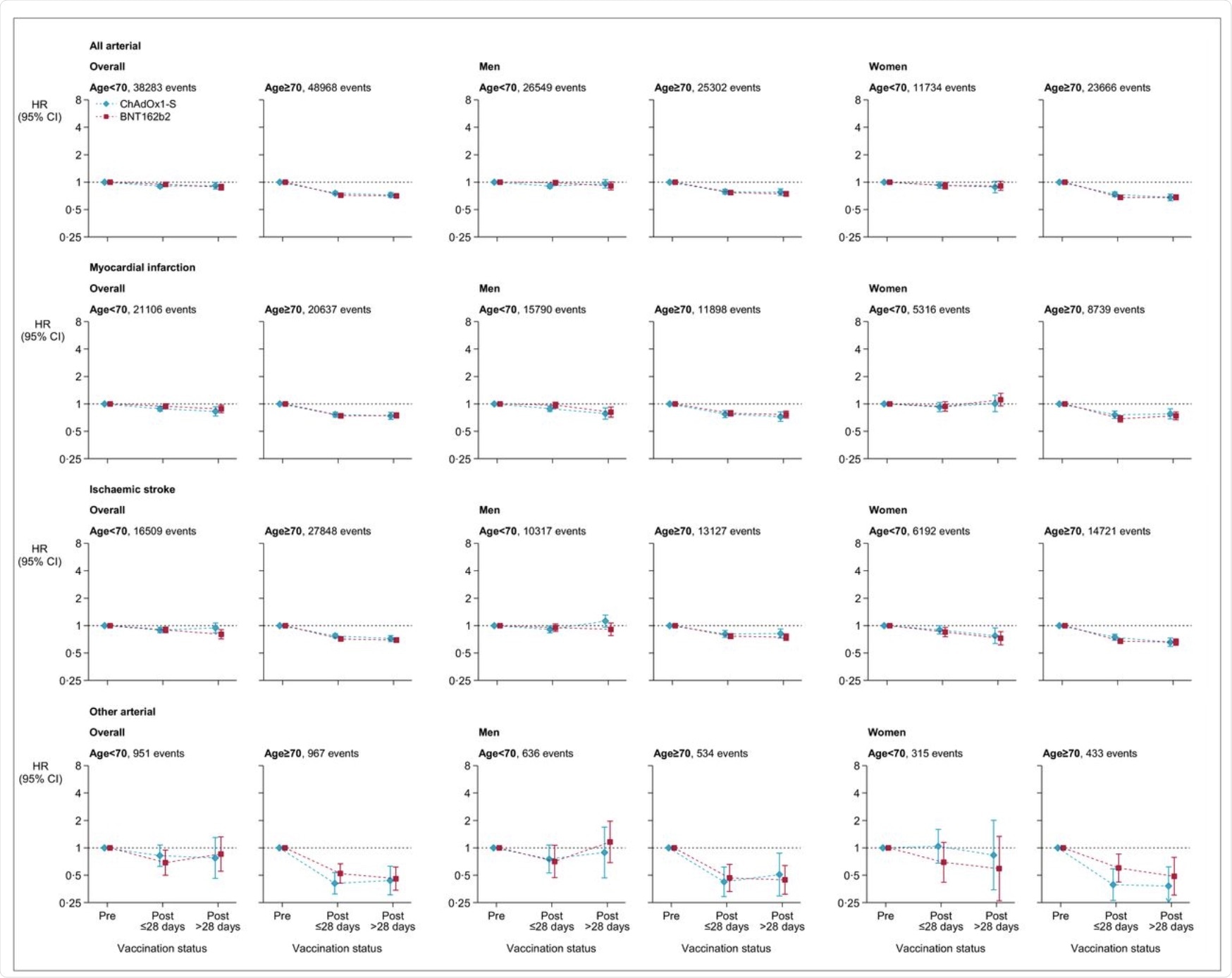 Adjusted hazard ratios for all arterial thromboses, myocardial infarction, ischemic stroke and other arterial thromboses after ChAdOx1-S or BNT162b2 vaccine.
Adjusted hazard ratios for all arterial thromboses, myocardial infarction, ischemic stroke and other arterial thromboses after ChAdOx1-S or BNT162b2 vaccine.
The rates of intracranial venous thrombosis (ICVT) and thrombocytopenia in individuals under the age of 70 years were found to be higher 1-28 days after the ChAdOx1-S vaccine dose. However, the rates of both thrombocytopenia and ICVT were not increased after the BNT162b2 vaccine. Both of these rates were in comparison to the pre-vaccinated ratios.
It was also found that the similar absolute excess risks of ICVT 1-28 days after the injection of the ChAdOx1-S vaccine were 0.9–3 per million, differing by age and sex of the patients.
Conclusion
In the current study, vaccination with ChAdOx1-S was associated with approximately 20fold higher rates of ICVT and hospitalization due to thrombocytopenia in patients under the age of 70, even after adjusting for their demographic characteristics and comorbidities. This same risk was not observed following BNT162b2 vaccination.
However, it should be noted that cases and risks of ICVT and thrombocytopenia after the vaccination of ChAdOx1-S were smaller as compared to its potential in reducing the severity of COVID-19 morbidity and mortality. The study also showed that the arterial or venous thrombosis rates were generally lower for people of or below 70 years of age after receiving either of the vaccine’s first dose.
Overall, the current study could be improved by focusing on patients younger than 40 years to get a better understanding of their risk of thrombotic events after receiving these vaccinations.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Whiteley, W. N., Ip, S., Cooper, J. A., et al. (2021). Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, and thrombocytopenic events: a whole population cohort study in 46 million adults in England. medRxiv. doi:10.1101/2021.08.18.21262222. https://www.medrxiv.org/content/10.1101/2021.08.18.21262222v1
- Peer reviewed and published scientific report.
Whiteley, William N., Samantha Ip, Jennifer A. Cooper, Thomas Bolton, Spencer Keene, Venexia Walker, Rachel Denholm, et al. 2022. “Association of COVID-19 Vaccines ChAdOx1 and BNT162b2 with Major Venous, Arterial, or Thrombocytopenic Events: A Population-Based Cohort Study of 46 Million Adults in England.” Edited by Suzanne C. Cannegieter. PLOS Medicine 19 (2): e1003926. https://doi.org/10.1371/journal.pmed.1003926. https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1003926.